Iron-based amorphous alloy having magnetothermal effect as well as application of iron-based amorphous alloy and method for regulating and controlling magnetic transition temperature of iron-based amorphous alloy
An iron-based amorphous alloy, magnetocaloric effect technology, applied in the direction of magnetic materials, magnetic objects, electrical components, etc., can solve the problems of inability to prepare into blocks, high Curie temperature, poor amorphous formation ability, etc.
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
Embodiment 1
[0047] In the present embodiment, the molecular formula of the iron-based amorphous alloy is (Fe 0.71 T m 0.05 B 0.24 ) 96 Nb 4 .
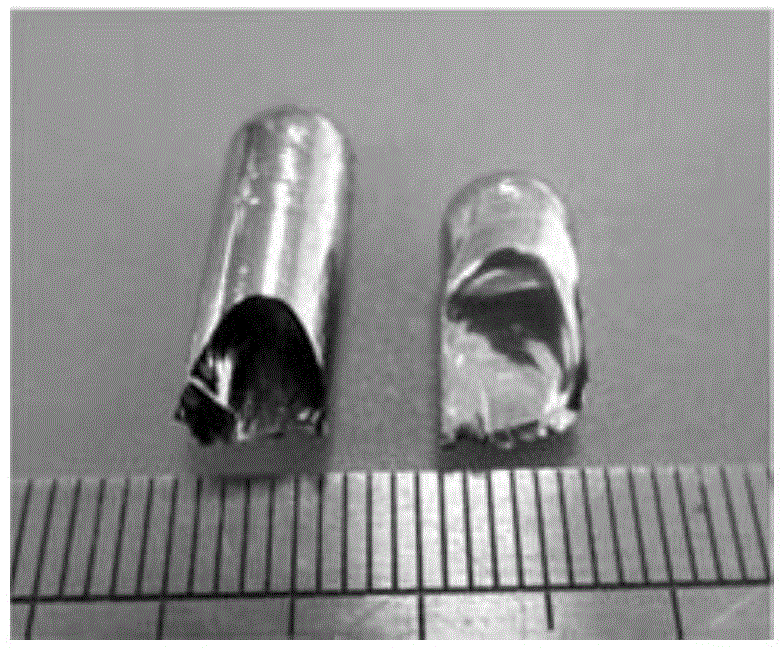
[0048] Utilize the following method to prepare the diameter of this (Fe 0.71 T m 0.05 B 0.24 ) 96 Nb 4 Bulk amorphous alloy round rod:
[0049] (1) Pure metal Fe, B, Nb, Tm elements with a purity of more than 99% are calculated according to the molecular formula (Fe 0.71 T m 0.05 B 0.24 ) 96 Nb 4 The atomic percentage in the preparation of raw materials;
[0050] (2) the raw material prepared by step (1) is placed in the water-cooled copper crucible of the electric arc melting furnace, and first vacuumized to 10 -5 Pa, then fill it with argon gas until the pressure is 600mbar for smelting, and then continue smelting for 5 minutes after melting, then let the alloy cool with the copper crucible until it solidifies, turn it over quickly, and repeatedly smelt 3 to 5 times to obtain a mother with uniform composition. alloy ingot;
[00...
Embodiment 2
[0060] In the present embodiment, the molecular formula of the iron-based amorphous alloy is (Fe 0.71 Er 0.05 B 0.24 ) 96 Nb 4 .
[0061] The (Fe 0.71 Er 0.05 B 0.24 ) 96 Nb 4 Bulk amorphous alloy rods. The preparation method is basically the same as that in Example 1, except that Er is used as the rare earth element in step 1, and the diameter of the cylindrical copper mold in step 3 is 5.5 mm.
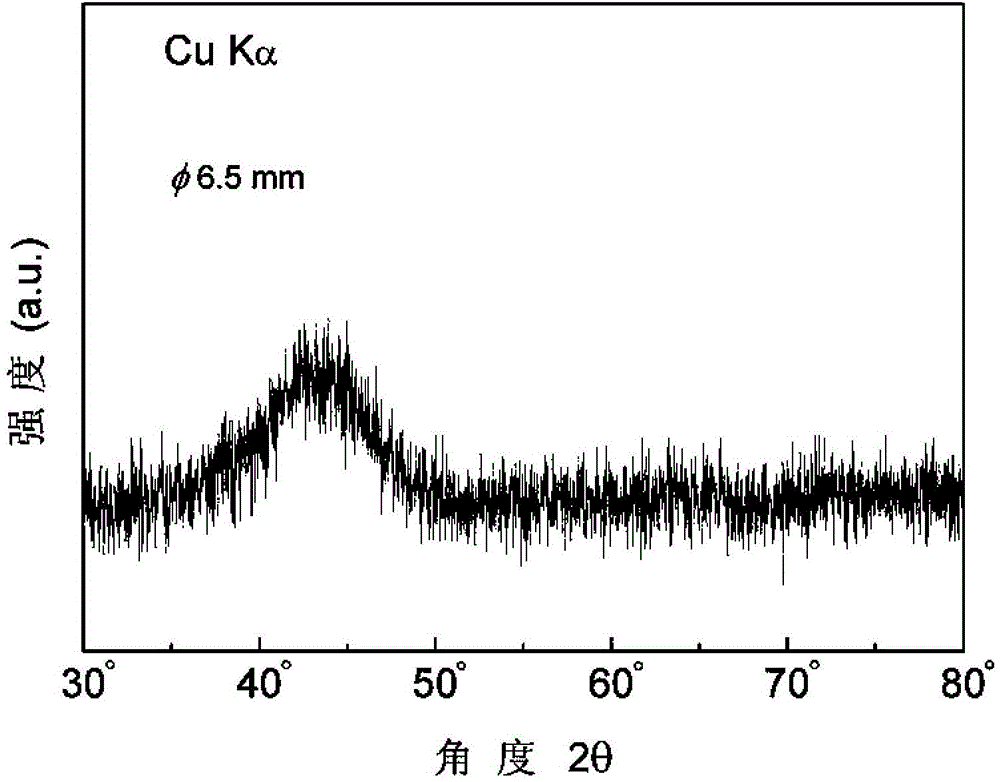
[0062] The X-ray diffraction pattern of the above-mentioned amorphous alloy bar made is as follows Figure 9 As shown, the rod has a broadened diffraction peak, indicating that the alloy rod is an amorphous structure.
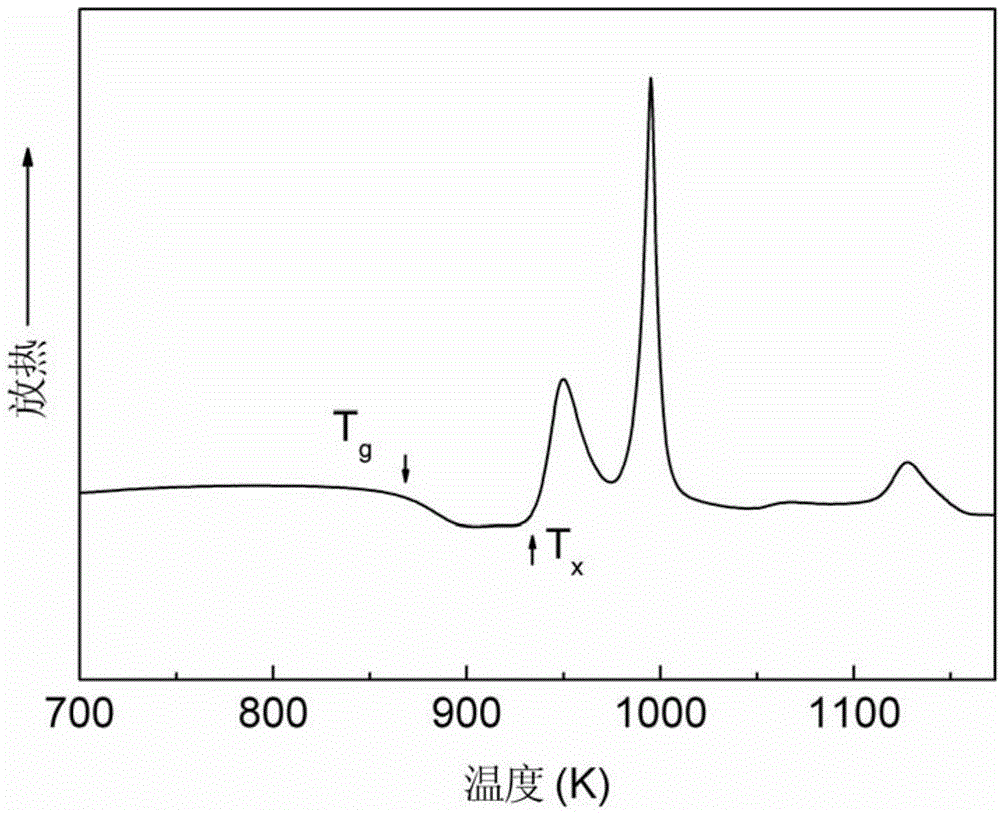
[0063] The thermodynamic parameters of the amorphous alloy rods prepared above were measured by differential scanning calorimetry. Its DSC heating curve is as follows Figure 10 shown.
[0064] It has been determined that the relationship between the change of magnetic entropy and temperature of the bulk amorphous alloy under an applied magnetic field of...
Embodiment 3
[0066] In the present embodiment, the molecular formula of the iron-based amorphous alloy is (Fe 0.71 Tb 0.05 B 0.24 ) 96 Nb 4 .
[0067] (Fe 0.71 Tb 0.05 B 0.24 ) 96 Nb 4 Bulk amorphous alloy rods. The preparation method is basically the same as that in Example 1, except that Tb is selected as the rare earth element in the batching in step 1, and the diameter of the cylindrical copper mold in step 3 is 3.5 mm.
[0068] The X-ray diffraction pattern of the above-mentioned amorphous alloy bar made is as follows Figure 9 As shown, the rod has a broadened diffraction peak, indicating that the alloy rod is an amorphous structure.
[0069] The thermodynamic parameters of the amorphous alloy rods prepared above were measured by differential scanning calorimetry. Its DSC heating curve is as follows Figure 10 shown.
[0070] It has been determined that the relationship between the change of magnetic entropy and temperature of the bulk amorphous alloy under an applied mag...
PUM
| Property | Measurement | Unit |
|---|---|---|
| diameter | aaaaa | aaaaa |
Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com