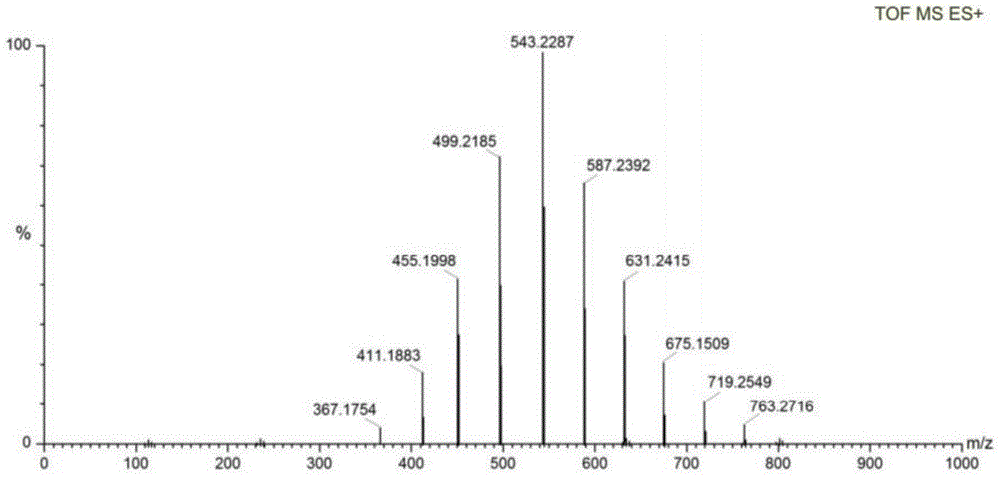
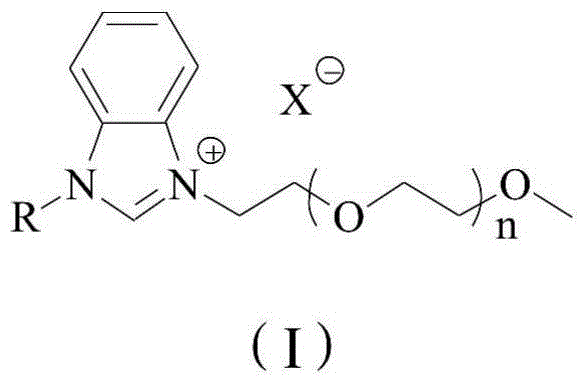
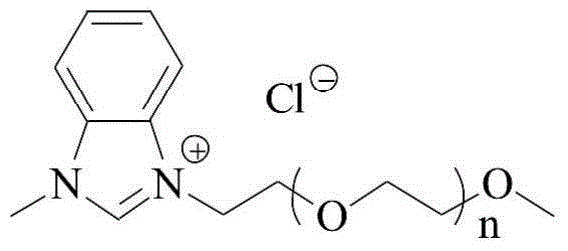
A kind of method using benzimidazole ionic liquid to prepare sartan biphenyl
A technology of benzimidazole and ionic liquid, applied in the field of preparation of sartan biphenyl, can solve the problems of high toxicity, high cost, low yield and the like, and achieve the effect of strong electron donating ability
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
Embodiment 1
[0028] The method for preparing sartan biphenyl using polyethylene glycol functionalized benzimidazole ionic liquid described in this embodiment comprises the following steps:
[0029] (1) Under nitrogen protection, add 2.4 mg of nickel chloride hexahydrate and 23.1 mg of polyethylene glycol-functionalized benzimidazole ionic liquid Ia to 8 mL of deionized water, and stir at 25° C. for 1 h to obtain a mixed solution;
[0030] (2) Add 137 mg of o-chlorobenzonitrile, 162 mg of 4-methylphenylboronic acid, and 80 mg of sodium hydroxide in sequence to the mixed solution, and stir at 100°C for reaction. After 4 hours, the reaction is completed, and the reaction solution is cooled to room temperature. , the reaction solution was extracted with ethyl acetate, the organic phase was collected, the organic phase was concentrated, the residue was separated by column chromatography, the eluent was petroleum ether: ethyl acetate=99:1 (v / v), and white 191 mg of solid is the target product, s...
Embodiment 2
[0040] The method for preparing sartan biphenyl using polyethylene glycol functionalized benzimidazole ionic liquid described in this embodiment comprises the following steps:
[0041] (1) Under nitrogen protection, add 1.2 mg of nickel chloride hexahydrate and 34.8 mg of polyethylene glycol-functionalized benzimidazole ionic liquid Ib to 10 mL of deionized water, and stir at 20° C. for 0.5 h to obtain a mixed solution;
[0042] (2) Add 182 mg of o-bromoxynil, 202 mg of 4-methylphenylboronic acid and 690 mg of potassium carbonate in sequence to the mixed solution, stir and react at 90°C, the reaction is completed after 7 hours, and the reaction solution is cooled to room temperature, The reaction liquid was extracted with ethyl acetate, the organic phase was collected, and the organic phase was concentrated, and the residue was separated by column chromatography, the eluent was petroleum ether: ethyl acetate=99:1 (v / v), and a white solid was obtained 185 mg, which is the targe...
Embodiment 3
[0050] The method for preparing sartan biphenyl using polyethylene glycol functionalized benzimidazole ionic liquid described in this embodiment comprises the following steps:
[0051] (1) Under nitrogen protection, add 1.2 mg of nickel chloride hexahydrate and 25.8 mg of polyethylene glycol-functionalized benzimidazole ionic liquid Ic to 10 mL of deionized water, and stir at 20° C. for 0.5 h to obtain a mixed solution;
[0052] (2) Add 182 mg of o-bromoxynil, 202 mg of 4-methylphenylboronic acid and 690 mg of potassium carbonate in sequence to the mixed solution, stir and react at 90°C, the reaction is completed after 7 hours, and the reaction solution is cooled to room temperature, The reaction liquid was extracted with ethyl acetate, the organic phase was collected, and the organic phase was concentrated, and the residue was separated by column chromatography, the eluent was petroleum ether: ethyl acetate=99:1 (v / v), and a white solid was obtained 189 mg, which is the targe...
PUM
 Login to View More
Login to View More Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com