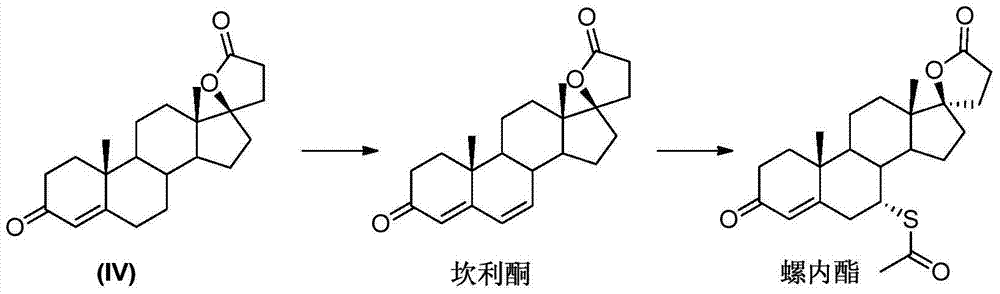
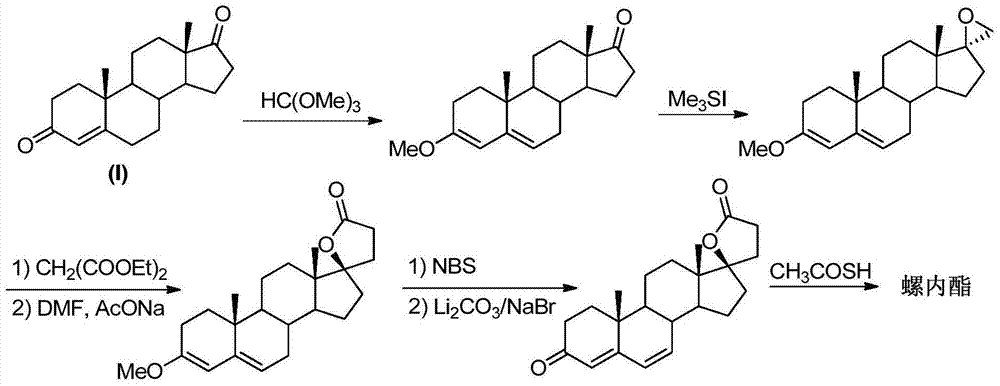
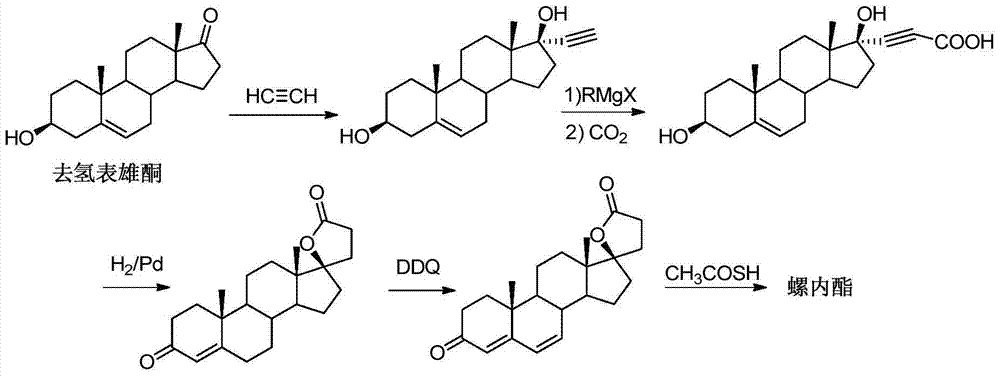
Synthesis method of spironolactone intermediate testosterone lactone
A testosterone lactone and synthesis method technology, applied in the field of chemical drug synthesis, can solve the problems of cumbersome operation, many reaction steps, and low yield, and achieve the effects of simple post-processing, easy operation, and high yield
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
Embodiment 1
[0032] Embodiment 1: addition deprotection reaction
[0033] The reaction formula is as follows:
[0034]
[0035] In reaction flask A, under nitrogen protection, add 100mL dry tetrahydrofuran (after strict anhydrous treatment KF<0.05%), 60mL n-butyllithium (1.6M n-hexane solution), and cool to -60°C. Slowly add (3-bromopropoxy)trimethylsilane (12.6 g), keep stirring at -60°C for 30 minutes, and set aside.
[0036]In reaction flask B, under the protection of nitrogen, the dried compound I (3 g) was dissolved in 30 mL of tetrahydrofuran (after strict anhydrous treatment KF<0.05%), and cooled to -40 °C. The pre-prepared LDA (12 mL, 2M tetrahydrofuran solution) was slowly added dropwise to the aforementioned reaction system, kept at -40° C. and stirred for 10 minutes, and set aside.
[0037] The solution system in reaction bottle A was slowly added dropwise to the solution system in reaction bottle B, warmed up to room temperature and reacted for 1 hour, TLC showed that the ...
Embodiment 2
[0038] Embodiment 2: addition deprotection reaction
[0039] In reaction bottle A, under nitrogen protection, add 100mL dry tetrahydrofuran (after strict anhydrous treatment KF<0.05%), 65mL sec-butyllithium (1.3M solution in cyclohexane), and cool to -78°C. Slowly add (3-bromopropoxy)triethylsilane (13.2 g), keep stirring at -60°C for 30 minutes, and set aside.
[0040] In reaction flask B, under the protection of nitrogen, the dried compound I (3 g) was dissolved in 30 mL of tetrahydrofuran (after strict anhydrous treatment KF<0.05%), and cooled to -40 °C. The pre-prepared LDA (12mL, 2M solution in tetrahydrofuran) was slowly added dropwise to the aforementioned reaction system, kept at -40°C and stirred for 10 minutes, and set aside.
[0041] The solution system in reaction bottle A was slowly added dropwise to the solution system in reaction bottle B, warmed up to room temperature and reacted for 1 hour, TLC showed that the reaction was complete, and 80 mL of water was add...
Embodiment 3
[0042] Example 3: Oxidative cyclization reaction
[0043] The reaction formula is as follows:
[0044]
[0045] Dissolve 10g of compound (II) in 150mL of dichloromethane, add 0.05g of 2,2,6,6-tetramethylpiperidine-1-oxyl radical (TEMPO) and 7g of potassium bromide in sequence, add 40mL of 10% Sodium hypochlorite aqueous solution and 0.5g phase transfer catalyst tetrabutylammonium chloride were stirred at 10-15°C for about 6 hours. After the reaction was complete, sodium sulfite solution (9g / 9mL water) was added to neutralize the oxidant, and the water layer was separated. Concentrate until there is no dichloromethane, add 30mL methanol and 10mL 10% dilute hydrochloric acid, stir at 20-25°C for 0.5 hours, neutralize with 0.5N sodium hydroxide solution to pH about 6.5, reduce the pressure to no methanol, add 100mL water, stir After 0.5 hour, filter and dry at 60° C. for 24 hours to obtain 8.7 g of testosterone lactone (III), the mass yield is about 87%, and the HPLC purity i...
PUM
 Login to View More
Login to View More Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com