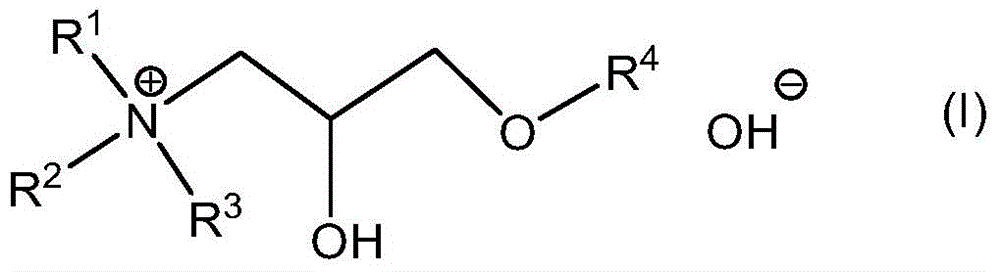
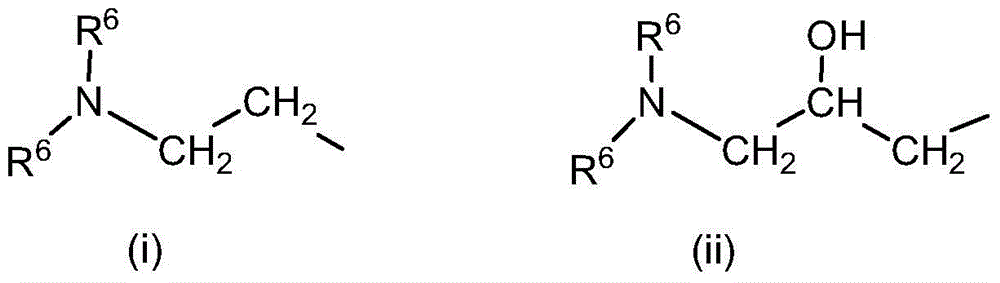
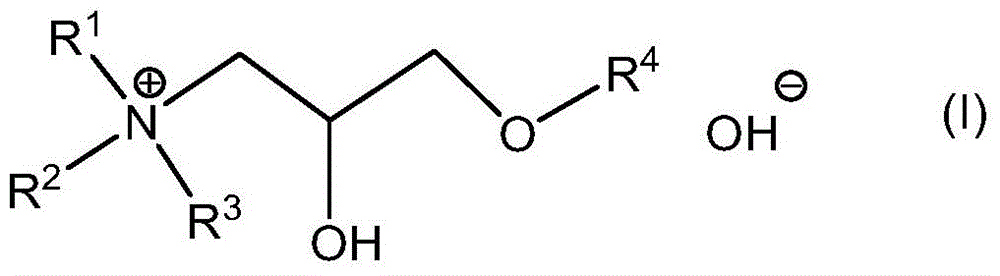
Quaternary ammonium hydroxide
一种季铵氢氧化物、残余物的技术,应用在阳离子表面活性化合物、有机化学等方向,能够解决组合物不能令人满意等问题
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
preparation example Construction
[0141] Preparation of Exemplary Quaternary Ammonium Hydroxides
[0142] Synthetic routes to these compounds should be straightforward and obvious to anyone skilled in the art of organic synthesis. These methods include, but are not limited to (1) the reaction between tertiary amines and ethylene oxide, as exemplified by the industrial synthesis of choline hydroxide disclosed in U.S. Patent No. 2,774,759:
[0143]
[0144] or (2) by metathesis by quaternary ammonium halides:
[0145]
[0146] Or (3) by electrodialysis from quaternary ammonium halides.
[0147] These quaternary ammonium hydroxides are available in essentially dry form, but they are readily soluble in DA solvents without the use of protic co-solvents. Furthermore, as described herein, they are effective as a source of hydroxide in place of TMAH in formulations intended for use as strippers and cleaners.
Embodiment 1
[0149]
[0150] 59.06 grams of a 25% by weight solution of trimethylamine (0.250 moles of trimethylamine) in methanol were weighed into a polypropylene reaction vessel. To this solution was added 14.50 g of isopropyl glycidyl ether (0.125 mol) via syringe under a nitrogen atmosphere with vigorous stirring (Teflon-coated magnetic stir bar). A strong exotherm was noticed immediately after starting the addition of the glycidyl ether to the amine solution. With the aid of a syringe pump, the rate of addition was adjusted to keep the internal temperature below about 10 degrees Celsius above ambient. When the glycidyl ether addition was complete, the temperature began to decrease slowly. The solution was stirred for an additional 30 minutes, during which time the internal temperature dropped by approximately 4°C.
[0151] At this point, the internal temperature is still higher than the ambient temperature. Add the solution containing 6.76 g of H via a syringe at a rate such th...
Embodiment 2
[0154] The methanol solution obtained in a part of Example 1 (about 7.5 grams, containing 8.8 × 10 -3 Molar quaternary ammonium hydroxide) was evaporated to a viscous oil in a 50 mL polypropylene centrifuge tube under a stream of dry nitrogen. The test tube containing the viscous oil is placed in a vacuum chamber and heated at -2 mm Hg was maintained for 5 hours during which time the viscous oil solidified as the last traces of solvent were removed.
[0155] Neat DMSO (12.84 g) was added to the tubes immediately after removal from the vacuum chamber, the caps were secured tightly and the contents of the tubes were stirred gently. After less than 5 minutes, all solids dissolved, resulting in a clear, colorless solution. Analysis of the solution by titration indicated an OH- concentration of 0.54 mol / kg (target 0.58 mol / kg). The water content by Karl-Fischer titration was zero. The dry, concentrated solution was effective for removing novolak photoresist residue from wafer co...
PUM
| Property | Measurement | Unit |
|---|---|---|
| purity | aaaaa | aaaaa |
Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com