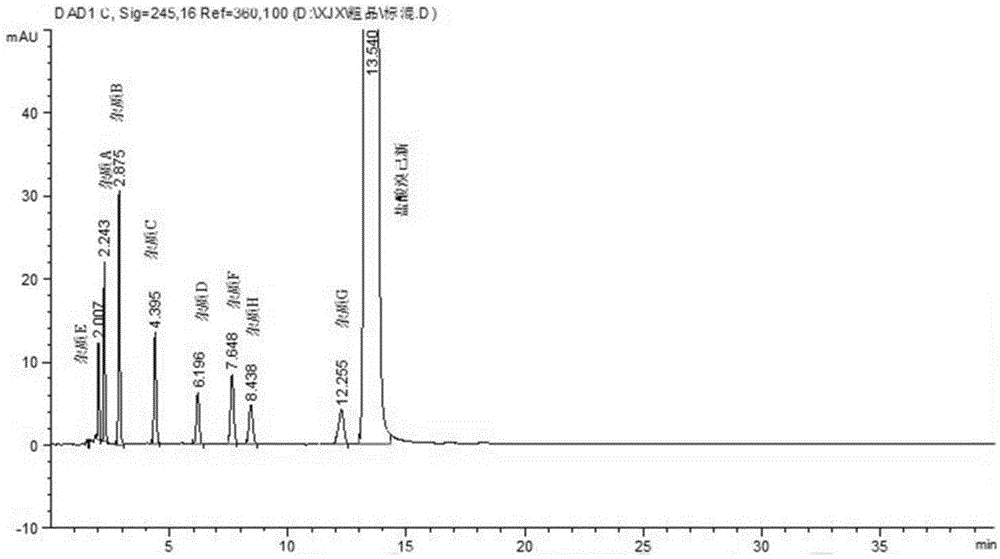
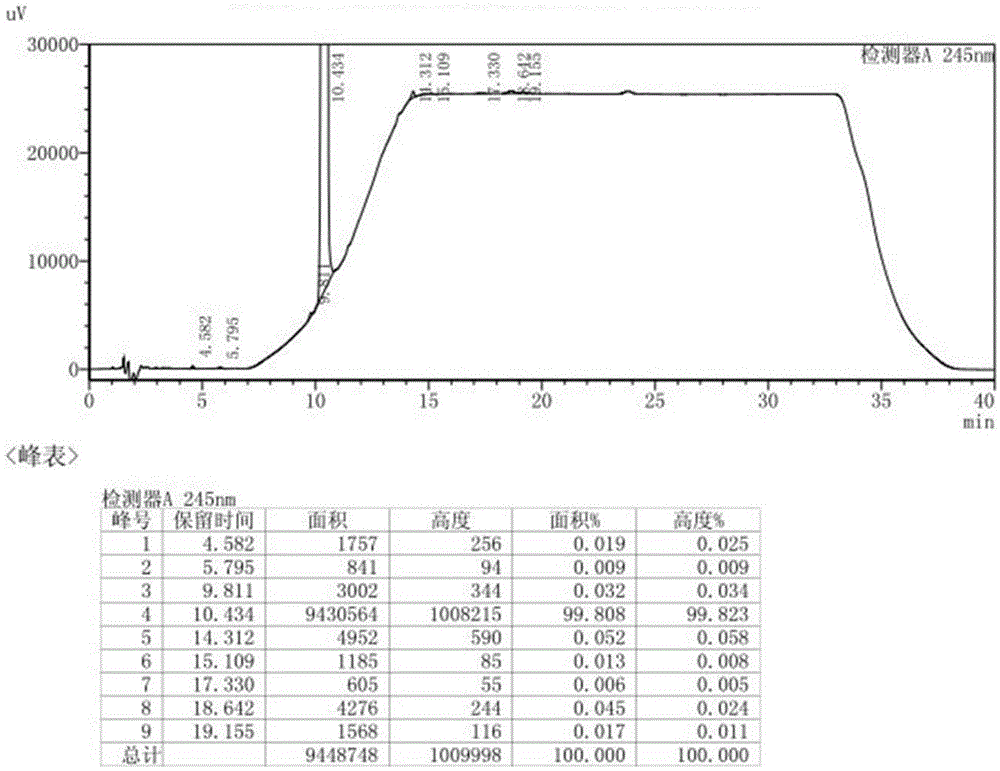
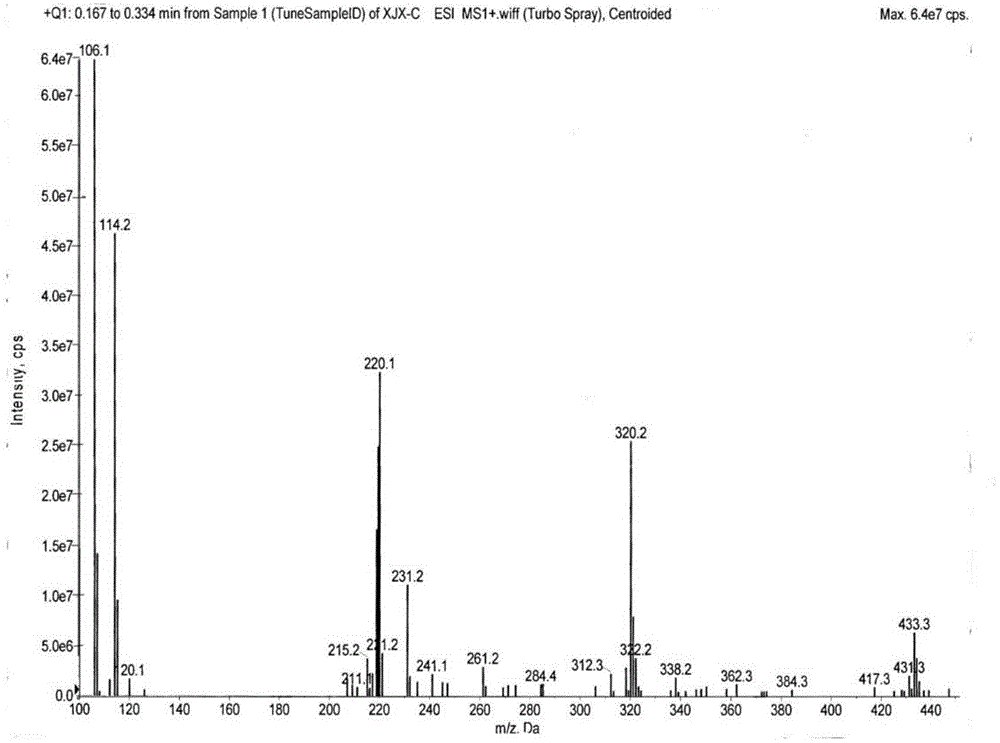
Preparation process of impurities contained in expectorant drug bromhexine hydrochloride
A technology of bromhexine hydrochloride and preparation process is applied in the preparation of organic compounds, the preparation of amino compounds, the preparation of amino-substituted functional groups, etc., and can solve problems such as low purity and high price
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
Embodiment 1
[0076] The preparation process of the impurity contained in the expectorant medicine bromhexine hydrochloride of embodiment 1
[0077] (1) Preparation of impurity C
[0078]
[0079] ①The preparation of intermediate anthranilic acid chloride
[0080] Add 5mL of thionyl chloride into a 10mL reaction flask, cool down to 10-20°C, add 1g of anthranilyl alcohol, raise the temperature to 40°C and stir for 1 hour, TLC monitors that the reaction is complete, distill off the thionyl chloride, and the obtained solid is 35°C Vacuum drying for 2 hours gave the target anthranilic acid chloride as a yellow solid;
[0081] ② Impurity C, namely the preparation of N-methyl-N-cyclohexyl-2-aminobenzylamine
[0082] Add 1mL of ethyl acetate and 1.1g of N-methylcyclohexylamine into a 10mL reaction flask, add 0.75g of anthraniloyl chloride, stir and react at 35°C for 2h after the addition, add 2mL of absolute ethanol, heat up and reflux for 0.5h , filtered while hot, the filtrate was rotary e...
Embodiment 2
[0129] The preparation technology of the impurity contained in embodiment 2 expectorant medicine bromhexine hydrochloride
[0130] (1) Preparation of impurity C
[0131]
[0132] ①The preparation of intermediate anthranilic acid chloride
[0133]Add 5mL of phosphorus oxychloride into a 10mL reaction flask, cool down to 10-20°C, add 1g of anthranilobenzyl alcohol, raise the temperature to 40°C and stir for 1 hour, TLC monitors that the reaction is complete, evaporate the phosphorus oxychloride, and the obtained solid is 35°C Vacuum drying for 2 hours gave the target anthranilic acid chloride as a yellow solid;
[0134] ② Impurity C, namely the preparation of N-methyl-N-cyclohexyl-2-aminobenzylamine
[0135] Add 1mL of ethanol and 1.1g of N-methylcyclohexylamine into a 10mL reaction flask, add 1.5g of anthraniloyl chloride, stir and react at 30°C for 2h after the addition, add 2mL of absolute ethanol, heat up and reflux for 0.5h, and Filtrate hot, remove the solvent by rot...
Embodiment 3
[0165] The preparation process of the impurity contained in embodiment 3 expectorant medicine bromhexine hydrochloride
[0166] (1) Preparation of impurity C
[0167]
[0168] ①The preparation of intermediate anthranilic acid chloride
[0169] Add 5mL of phosphorus trichloride into a 10mL reaction flask, cool down to 10-20°C, add 1g of anthranilobenzyl alcohol, raise the temperature to 40°C and stir the reaction for 1h, TLC monitors that the reaction is complete, evaporate the phosphorus trichloride, and the obtained solid is 35°C Vacuum drying for 2 hours gave the target anthranilic acid chloride as a yellow solid;
[0170] ② Impurity C, namely the preparation of N-methyl-N-cyclohexyl-2-aminobenzylamine
[0171] Add 1mL of DMSO and 1.1g of N-methylcyclohexylamine into a 10mL reaction flask, add 0.37g of anthraniloyl chloride, stir and react at 35°C for 2h after the addition is complete, add 2mL of absolute ethanol, heat up and reflux for 0.5h, while hot Filtrate, remove...
PUM
 Login to View More
Login to View More Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com