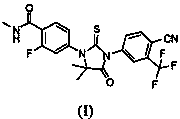
Novel Xtandi crystal form and preparation method thereof
A technology of enzalutamide and crystal form, applied in the field of medicinal chemistry, can solve the problem of not mentioning the spectral characteristics of B1 crystal form enzalutamide, and achieve the effects of good solubility and stable properties
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
Embodiment 1
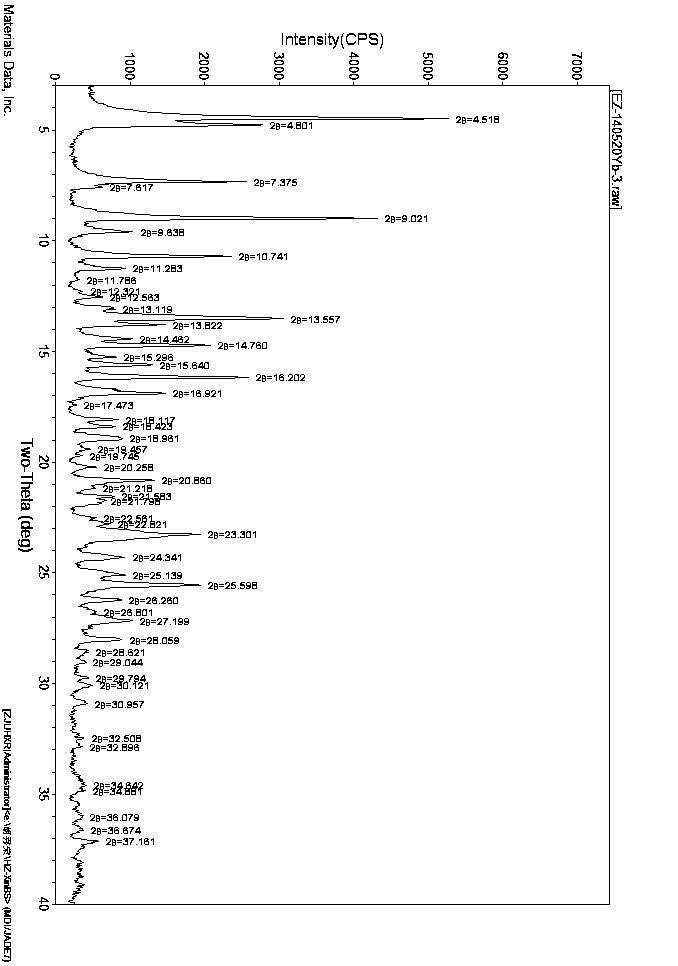
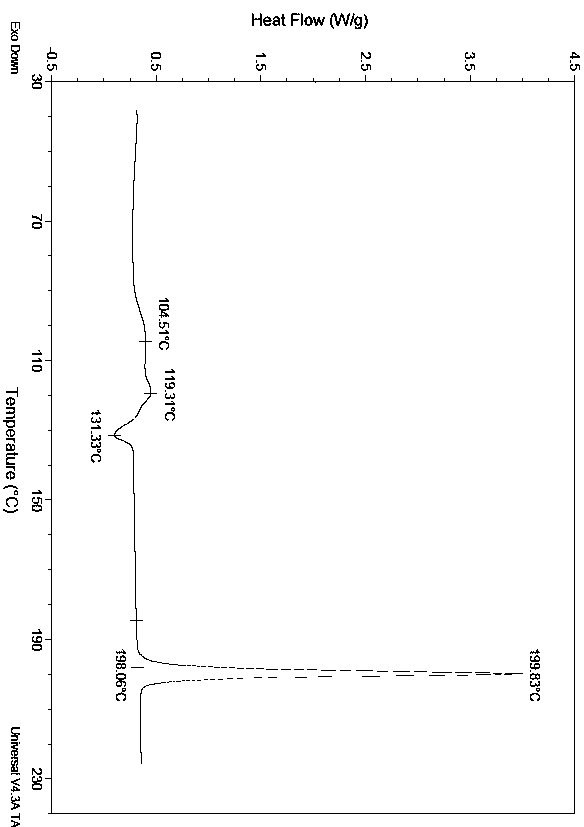
[0026] Take 2.0g of enzalutamide solid, add 6ml of mixed solvent (DMSO:toluene=1:2), heat to 90°C, heat to dissolve, cool to 50°C, dilute with 8ml of isopropyl acetate, dilute the diluted solution with 8ml of saturated saline Wash twice, then dry with anhydrous sodium sulfate and filter. The filtrate is spin-dried at 50°C. The oily matter is slurried with 20ml of isopropanol for 16 hours and then filtered. The filter cake is placed in the air at room temperature for 24 hours and then 60°C. After vacuum drying, 1.91 g of enzalutamide in B1 crystal form was obtained, with a yield of 95.5%.
Embodiment 2
[0028] Take 1.2g of enzalutamide solid, add 6ml of mixed solvent (DMSO:1,4-dioxane=1:2) and heat to 90°C to dissolve, then cool to 60°C, add 4ml of 1,4-dioxane After diluting the hexacyclone with 6ml of ethyl acetate, the diluted solution was washed three times with 6ml of water, then dried over anhydrous sodium sulfate and filtered, the filtrate was spin-dried at 60°C, and the oil was mixed with 6ml of a solvent (isopropanol:DMSO=10:1 ) was heated and dissolved at 75°C, cooled to room temperature, filtered, and the filter cake was air-dried at room temperature for 24 hours and then vacuum-dried at 60°C to obtain 1.02 g of enzalutamide in B1 crystal form, with a yield of 85%.
Embodiment 3
[0030] Take 2.0g of enzalutamide solid, add 10ml of mixed solvent (DMSO: isopropyl acetate = 1:4), heat to 80°C to dissolve, cool to 50°C, dilute with 12ml of isopropyl acetate, wash with 12ml of saline Wash twice, then dry with anhydrous sodium sulfate and filter. The filtrate is spin-dried at 60°C. The oily matter is slurried with 20ml of isopropanol for 16 hours and then filtered. The filter cake is placed in air at room temperature for 24 hours and then 60°C. After vacuum drying, 1.86 g of enzalutamide in crystal form B1 was obtained, with a yield of 93%.
PUM
 Login to View More
Login to View More Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com