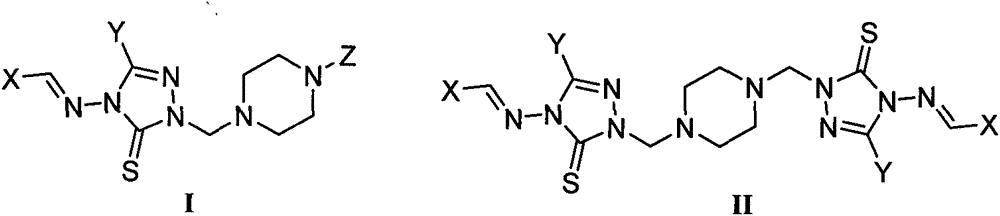
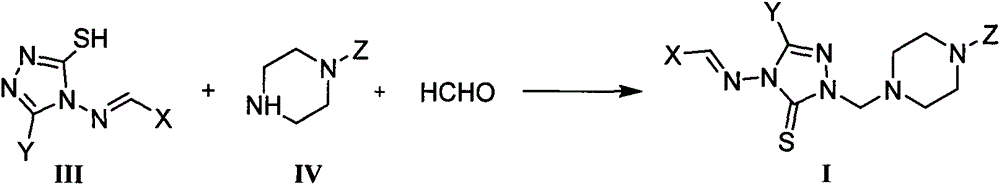
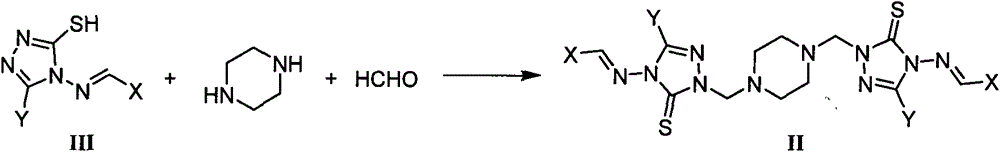1,2,4-triazole mannich base derivatives containing substituted piperazidine, and preparation method and application thereof
A derivative, piperazine technology, applied in botany equipment and methods, chemicals for biological control, applications, etc., can solve the problems of preparation of bactericidal and herbicidal activities that have not been disclosed, and achieve good living control effect, high The effect of bactericidal activity and high in vitro inhibitory activity
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
Embodiment 1
[0026] Preparation method of compound 17.
[0027] Step A: Preparation of (E)-5-methyl-4-((thiophene-2-methylene)amino)-4H-1,2,4-triazole-3-thiol
[0028]
[0029] Add 5.2g (0.04mol) 4-amino-5-methyl-4H-1,2,4-triazole-3-thiol and 5.1g (0.045mol) 2-thiophenecarbaldehyde to a 100mL round bottom flask, add 30mL of Dissolve it in absolute ethanol, then add 7-8 drops (about 0.5 mL) of glacial acetic acid dropwise, and heat to reflux for 5 hours. After cooling to room temperature, crystals were precipitated, filtered with suction, washed with a small amount of ethanol, and dried to obtain 5.46 g of brown crystals, with a yield of 61.2%.
[0030] Step B: Preparation of (E)-5-methyl-2-((4-phenylpiperazin-1-yl)methyl)-4-((thiophene-2-methylene)amino)-2,4 -Dihydro-3H-1,2,4-triazole-3-thione (compound 17)
[0031]
[0032] In a 50 mL round bottom flask, add 0.336 g (1.5 mmol) of (E)-5-methyl-4-((thiophene-2-methylene)amino)-4H-1,2,4-triazole-3 -thiol, 0.243g (1.5mmol) phenylpip...
Embodiment 2
[0034] Preparation method of compound 29.
[0035] Step A: Preparation of (E)-4-(((5-chloro-1,3-dimethyl-1H-pyrazol-4-yl)methylene)amino)-5-methyl-4H-1, 2,4-triazole-3-thiol
[0036]
[0037] Add 1.30g (0.01mol) 4-amino-5-methyl-4H-1,2,4-triazole-3-thiol and 1.59g (0.01mol) 5-chloro-1,3- Add 12 mL of glacial acetic acid to dimethyl-1H-pyrazole-4-carbaldehyde, and heat to reflux for 1 hour. Cool to room temperature, filter with suction, wash with a small amount of ethanol, and dry to obtain 2.37 g of white solid, with a yield of 87.6%.
[0038] Step B: Preparation of (E)-2-((4-benzylpiperazin-1-yl)methyl)-4-(((5-chloro-1,3-dimethyl-1H-pyrazole-4 -yl)methylene)amino)-5-methyl-2,4-dihydro-3H-1,2,4-triazole-3-thione (compound 29)
[0039]
[0040]In a 50 mL round bottom flask, add 0.27 g (1 mmol) (E)-4-(((5-chloro-1,3-dimethyl-1H-pyrazol-4-yl)methylene)amino)- 5-methyl-4H-1,2,4-triazole-3-thiol, 0.176g (1mmol) benzylpiperazine, 20mL N,N-dimethylformamide, and finally 0....
Embodiment 3
[0042] Preparation method of compound 45.
[0043] Step A: Preparation of (E)-4-(((5-Chloro-3-methyl-1-phenyl-1H-pyrazol-4-yl)methylene)amino)-5-methyl-4H- 1,2,4-triazole-3-thiol
[0044]
[0045] Add 1.30g (0.01mol) 4-amino-5-methyl-4H-1,2,4-triazole-3-thiol and 2.21g (0.01mol) 5-chloro-3-methyl to 100mL round bottom flask -1-Phenyl-1H-pyrazole-4-carbaldehyde, add 20mL of glacial acetic acid, and heat to reflux for 5 hours. Cool to room temperature, filter with suction, wash with a small amount of ethanol, and dry to obtain 3.11 g of white solid with a yield of 93.5%.
[0046] Step B: Preparation of (E)-4-(((5-chloro-3-methyl-1-phenyl-1H-pyrazol-4-yl)methylene)amino)-5-methyl-2- ((4-(4-methylpyrimidin-2-yl)piperazin-1-yl)methyl)-2,4-dihydro-3H-1,2,4-triazole-3-thione (compound 45)
[0047]
[0048] In a 50 mL round bottom flask, add 0.30 g (0.9 mmol) of (E)-4-(((5-chloro-3-methyl-1-phenyl-1H-pyrazol-4-yl)methylene) Amino)-5-methyl-4H-1,2,4-triazole-3-thiol, 0.160g...
PUM
 Login to View More
Login to View More Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com