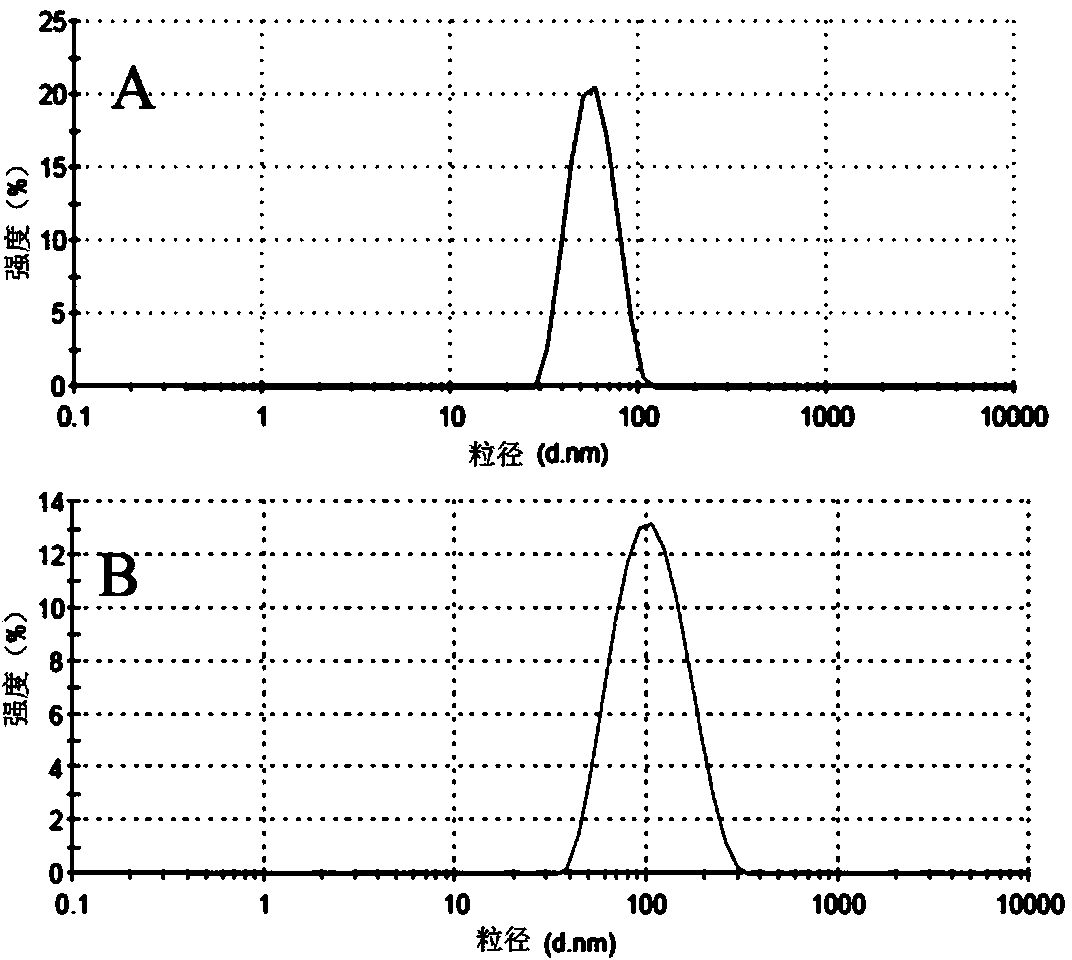
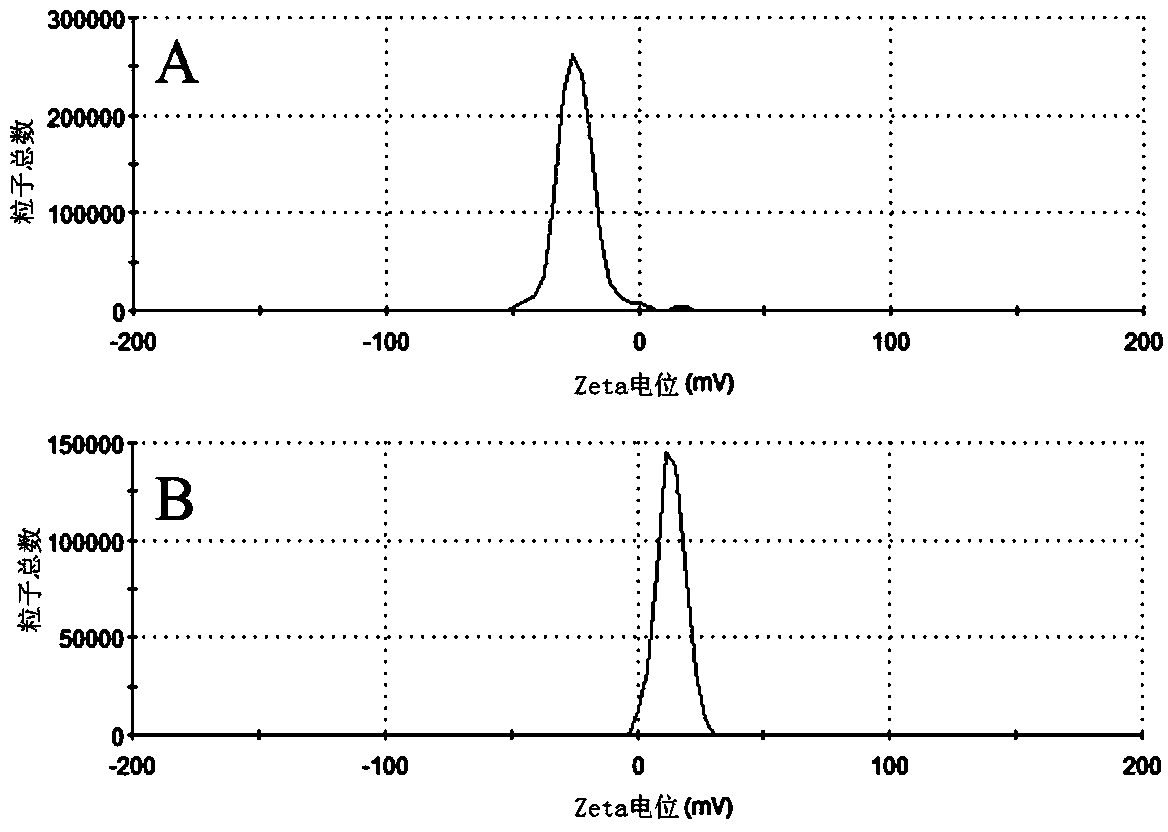
Preparation method of resveratrol-loaded and amino-modified mesoporous silica nanoparticles
A technology of mesoporous silica and amino modification, applied in the direction of active ingredients of hydroxyl compounds, medical preparations of non-active ingredients, pharmaceutical formulations, etc., can solve the problems of unstable physical and chemical properties, insoluble in water, short half-life, etc. Achieve the effect of improving bioavailability, good market prospects, and increasing drug loading
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
Embodiment 1
[0021] 1 Instruments and materials
[0022] 1.1 Instrument
[0023] Waters high performance liquid chromatograph (Waters e2695 quaternary pump, 2998 PDA detector, U.S. Waters company);
[0024] Nano-ZS 90 laser particle size analyzer (Malvern Instrument Co., Ltd., UK);
[0025] Optima MAX ultra-low temperature centrifuge (Beckman Coulter Co., Ltd., USA);
[0026] Mill-Q ultrapure water meter (Millpore, USA);
[0027] PH acidity meter (Switzerland Mettler-Toledo company);
[0028] JEM-1200EX transmission electron microscope (Japan JEOL company);
[0029] Vector 22 Fourier transform infrared spectrometer (Germany BRUKER company);
[0030] TRISTAR II3020 automatic specific surface and pore analyzer (Micromeritics Instrument Company, USA);
[0031] Rigaku D / max 2550PC automatic polycrystalline X-ray diffractometer (Japan Rigaku Electric Co., Ltd.);
[0032] Thermo Forma ultra-low temperature refrigerator (U.S. Thermo Fisher Scientific company);
[0033] TGL-16B high-speed ...
PUM
 Login to View More
Login to View More Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com