Method for synthesizing 3-methyl-3-butene-1-ol
A synthesis method and technology of butene, applied in chemical instruments and methods, preparation of hydroxyl compounds, preparation of organic compounds, etc., can solve the problem of low content of 3-methyl-3-butene-1-ol finished products and long holding time and other problems, to achieve the effects of less harsh reaction conditions, less waste, and high reaction selectivity
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
preparation example Construction
[0038] A kind of synthetic method of 3-methyl-3-butene-1-alcohol, comprises the following steps:
[0039] a, preparation of paraformaldehyde isopropanol solution
[0040] Throw paraformaldehyde and isopropanol into a single-necked flask with magnetic stirring, and stir at 50-90°C until all formaldehyde is dissolved to obtain a formaldehyde-isopropanol solution.
[0041] b. Add catalyst
[0042] Then add a certain amount of catalyst into the flask, continue to stir to dissolve it completely, and then cool down to room temperature to obtain the formaldehyde isopropanol solution in a one-necked bottle sealed for storage, and detect the formaldehyde content and catalyst content in the solution.
[0043] c. Reaction with isobutene
[0044] Take the formaldehyde isopropanol solution and isobutylene prepared in step b, fill it into an autoclave with mechanical stirring, the reaction pressure is 5-10 MPa, keep stirring at a speed of 200r / min and heat up;
[0045] When the temperatu...
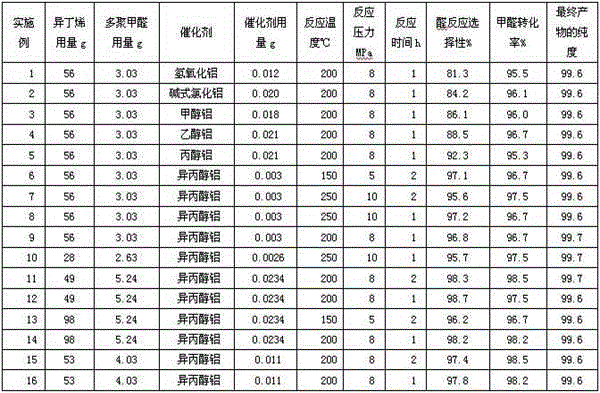
Embodiment 1
[0051] A kind of synthetic method of 3-methyl-3-buten-1-ol, throws 58g paraformaldehyde, 200g isopropanol in the single-necked flask with magnetic stirring, stirs at 50~70 ℃ until formaldehyde is all dissolved, A solution of formaldehyde in isopropanol was prepared.
[0052] Then add 0.20g of aluminum hydroxide to the flask as a catalyst and continue stirring to dissolve it completely, then cool down to room temperature to obtain a formaldehyde isopropanol solution and store it in a one-necked bottle. The formaldehyde content of the solution is 20.2%, and the aluminum hydroxide content is 0.08%. .
[0053] Take 15g of formaldehyde isopropanol solution and 56g of isobutene, fill them into an autoclave with mechanical stirring, the reaction pressure is 8MPa, keep stirring at a speed of 200r / min and start to heat up, when the temperature reaches 200°C, start timing and heat preservation reaction for 1 hour. After cooling down to room temperature, the autoclave was opened to slow...
Embodiment 2
[0057] A kind of synthetic method of 3-methyl-3-buten-1-ol, throws 58g paraformaldehyde, 200g isopropanol in the single-necked flask with magnetic stirring, stirs at 50~70 ℃ until formaldehyde is all dissolved, A solution of formaldehyde in isopropanol was prepared.
[0058] Then add 0.34g basic aluminum chloride to the flask and continue to stir to make it all dissolve, then cool down to room temperature to obtain formaldehyde isopropanol solution and store it in a one-necked bottle. The formaldehyde content of the solution is 20.2%, and the basic aluminum chloride content is 20.2%. 0.13%.
[0059] Take 15g of formaldehyde isopropanol solution and 56g of isobutylene, fill them into an autoclave with mechanical stirring, the reaction pressure is 8 MPa, keep stirring at a speed of 200r / min and start to heat up, when the temperature reaches 200°C, start timing and heat preservation reaction for 1 hour . After cooling down to room temperature, the autoclave was opened to slowly...
PUM
 Login to View More
Login to View More Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com