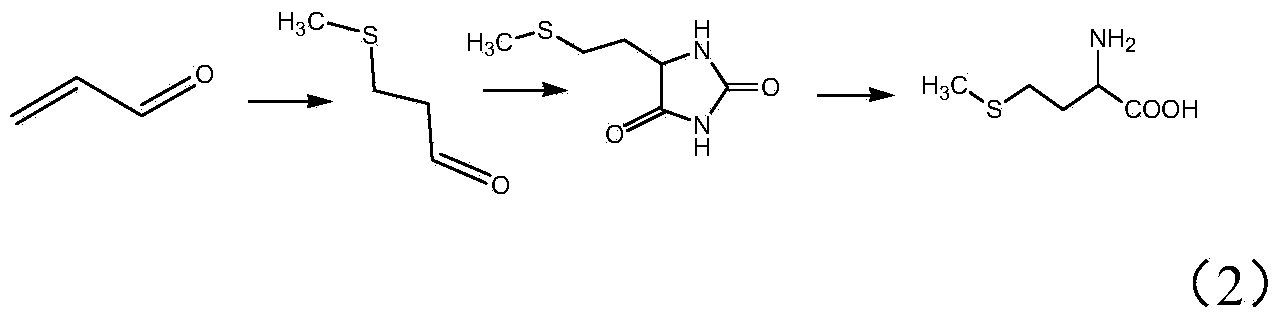
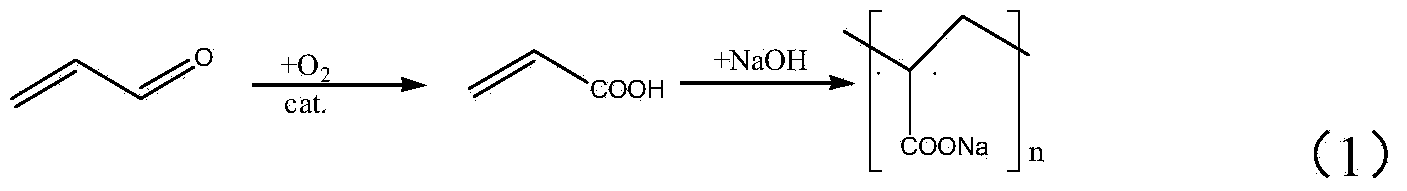Preparation method of acraldehyde
A technology for acrolein and crude glycerin, which is applied in the preparation of carbon-based compounds, chemical instruments and methods, and the preparation of organic compounds, etc., can solve the problems of high energy consumption, short life, and complicated process of purification operations, and achieve low glycerin purity requirements , reduce cost and energy consumption, and simplify the process flow
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
Embodiment 1
[0053] Example 1 (reaction effect comparison between desalted crude glycerin and crude glycerin)
[0054] This example compares the reaction effect of dehydration reaction to prepare acrolein with crude glycerol that has been desalted substantially as raw material and the reaction effect of dehydration reaction with crude glycerol as raw material.
[0055] Catalyst preparation: weigh 0.25g of Cs according to 20% load 2 CO 3 , and dubbed into 0.2mol L -1 concentration of aqueous solution, add 8g of SBA-15 carrier to the above aqueous solution, so that the loading capacity is 50%, stir vigorously for 2 to 5 hours, soak at room temperature overnight, dry at 80°C for later use, weigh an appropriate amount of heteropolyacid H 3 PW 12 o 40 12H 2 O and made into 0.08mol·L -1 Concentration in aqueous solution makes Cs x h 3-x PW 12 o 40 Where x=2.5 and mixed with the above-mentioned carrier, vigorously stirred for 2-5 hours by equal volume impregnation, then impregnated over...
Embodiment 2
[0061] Example 2 (desalted crude glycerin and refined glycerol are raw materials for reaction effect comparison)
[0062] This embodiment compares the reaction effect of desalted crude glycerin as raw material to prepare acrolein by dehydration reaction and that of refined glycerin as raw material.
[0063] Respectively adopt refined glycerol after water evaporation and concentration, desalination, removal of organic matter such as methanol, fatty acid and its esters, and basically only desalination treatment (for example, desalination treatment to a salt content lower than 1wt%), without other The desalted crude glycerol in the refining operation is used as raw material, and the catalytic dehydration reaction is carried out under the same conditions as other conditions; the preparation of the catalyst and the reaction conditions are the same as in Example 1. The results are compared in Table 2 below.
[0064] It can be seen from Table 2 that the conversion rate of glycerin r...
Embodiment 3
[0067] Embodiment 3 (glycerol containing different impurities is a raw material for reaction effect comparison)
[0068] Using the refined glycerol after water evaporation and concentration, desalting, methanol, fatty acid and its esters and other organic matter removal as raw material, add 2wt% methanol, 2wt% fatty acid, 2wt% fatty acid ester and 2wt% salt for reaction. Carry out sampling evaluation catalyst activity after continuous reaction 40h, selectivity and stability, catalyst composition and other reaction conditions are with embodiment example 1, and the result is compared in following table 3.
[0069] By comparing the reaction yields in Table 3, it can be seen that organic impurities such as methyl alcohol in crude glycerin, fatty acid, and fatty acid ester will not affect the catalyst reaction effect. The yield of acrolein is 88%, comparable to that of refined glycerin and desalted crude glycerin. However, the catalytic activity of refined glycerol containing salt...
PUM
 Login to View More
Login to View More Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com