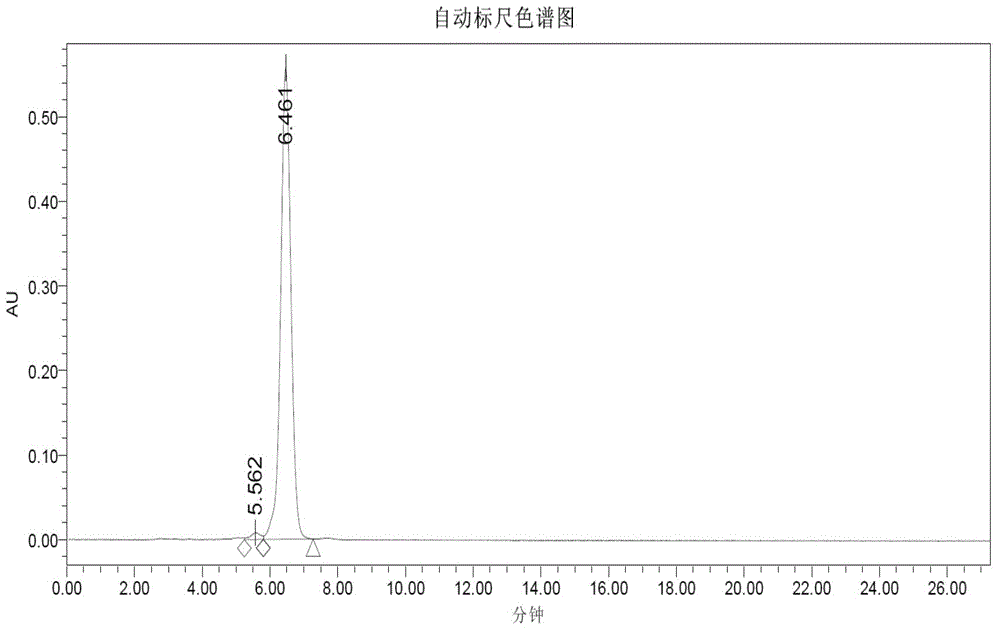
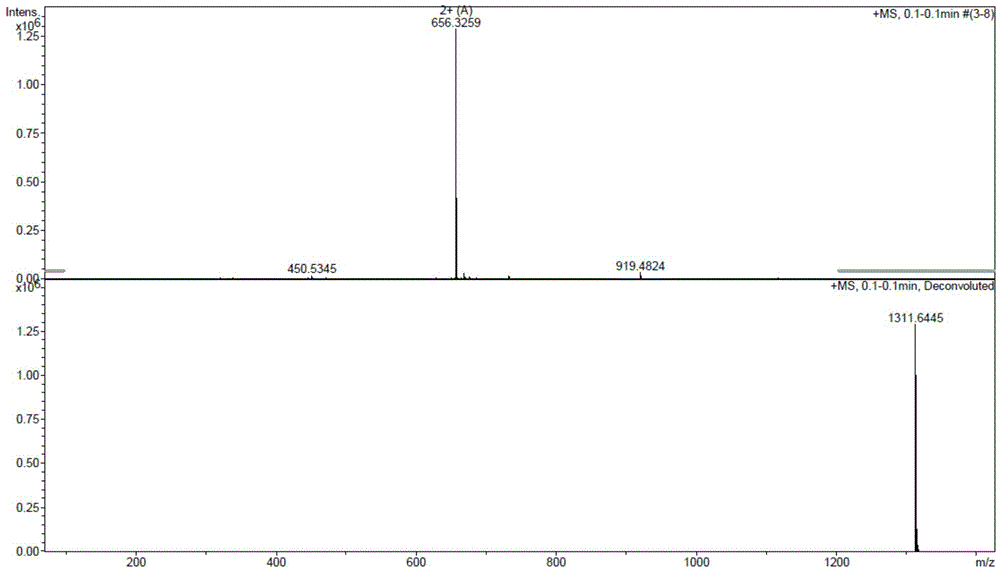
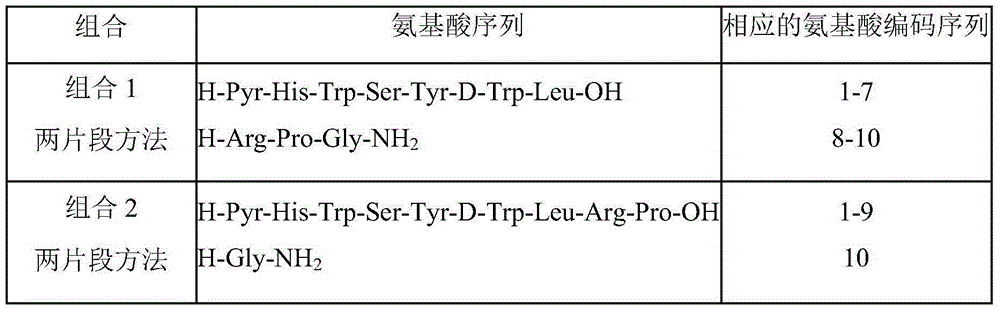
Method for preparing triptorelin by using fragment condensation
A technology for triptorelin acetate and fragments, applied in the field of pharmacy, can solve the problems of difficult triptorelin, difficult product purification, cost reduction, etc., and achieve the effects of low price, reduction of preparation times, and material cost saving
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
Embodiment 1
[0074] 1. Resin Preparation
[0075] Preparation of Fmoc-Leu-2-chloro-trityl resin: 2-chloro-trityl chloride resin (5 g, substitution value 0.84 mmol / g resin, 1 eq) was added to the polypeptide synthesizer, and the resin was washed with 60 mL of DCM. The solvent was drained and a solution of Fmoc-Leu-OH (1.3 eq) and DIEA (2.5 eq) in 30 mL of DCM was added. The mixture was mechanically stirred under an argon atmosphere for 1 hour. Add 10 mL of chromatographic methanol (2 ml / g resin) to block the active part on the resin for 30 minutes. The solvent was drained, washed with 3×50mL DMF, 3×50mL DCM, 3×50mL MeOH, and vacuum-dried to constant weight to obtain 5.82g of Fmoc-Leu-2-chloro-trityl resin. The amount of Fmoc in the piperidine deprotection solution was measured by ultraviolet spectrophotometry, and the loading amount of the resin was 0.52 mmol / g.
[0076] 2. Fragment Preparation
[0077] 2.1 Preparation of peptide fragment AA(1-7)-OH:
[0078] Add 5 g of Fmoc-Leu-2-chlo...
Embodiment 2
[0114] 1. Resin Preparation
[0115] Preparation of Fmoc-Pro-2-chloro-trityl resin: 2-chloro-trityl chloride resin (5 g, substitution value 0.84 mmol / g resin, 1 eq) was added to the polypeptide synthesizer, and the resin was washed with 60 mL of DCM. The solvent was drained and a solution of Fmoc-Pro-OH (1.3 eq) and DIEA (2.5 eq) in 30 mL of DCM was added. The mixture was mechanically stirred under an argon atmosphere for 1 hour. Add 10 mL of chromatographic methanol (2 ml / g resin) to block the active part on the resin for 30 minutes. The solvent was drained, washed with 3×50mL DMF, 3×50mL DCM, 3×50mL MeOH, and vacuum-dried to constant weight to obtain 5.69g of Fmoc-Pro-2-chloro-trityl resin. The amount of Fmoc in the piperidine deprotection solution was measured by ultraviolet spectrophotometry, and the loading amount of the resin was 0.46mmol / g.
[0116] 2. Fragment Preparation
[0117] Preparation of peptide fragment AA(1-9)-OH:
[0118] Add 5 g of Fmoc-Pro-2-chloro-tr...
PUM
 Login to View More
Login to View More Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D Engineer
- R&D Manager
- IP Professional
- Industry Leading Data Capabilities
- Powerful AI technology
- Patent DNA Extraction
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2024 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com