Application of labdane-type diterpene Leoleorin H in preparation of anti-tumor medicament
An anti-tumor drug, tumor technology, used in anti-tumor drugs, drug combinations, pharmaceutical formulations, etc.
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
Embodiment 1
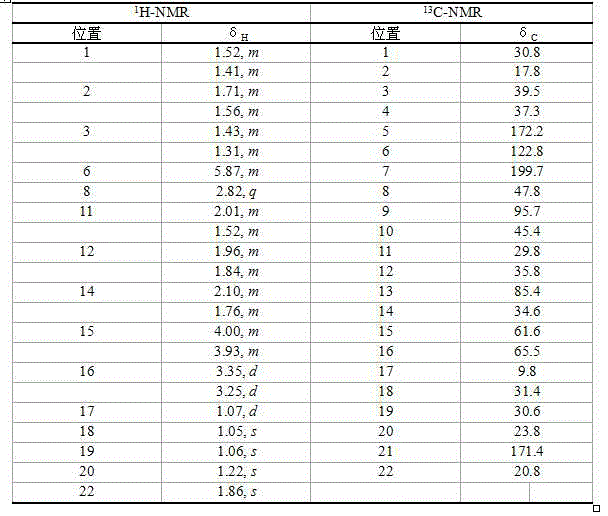
[0012] Embodiment 1: the preparation method of compound Leoleorin H
[0013] 5 kg of dry powder of the aerial part of Motherwort (purchased from Shanghai Kangqiao Chinese Medicine Decoction Pieces Co., Ltd., place of origin Anhui) was degreased by percolation method at room temperature for 3 times with petroleum ether, each volume 15 L; the dregs were extracted with acetone 3 times, each time The sub-volume is 15 L, and the acetone extracts are combined and recovered under reduced pressure to obtain 280 g of extract. The extract was dissolved in 300ml of a mixed solvent of dichloromethane and methanol (1:1, V / V), mixed with silica gel to mix the sample (500g, 200-300 mesh), and the evenly mixed silica gel was obtained after evaporating the solvent. Use 200-300 mesh silica gel (1500g) to pack the column and separate the mixed sample silica gel. Elute with n-hexane-ethyl acetate gradient (10:1, 4:1, 2:1, 1:1, 1:4, 0:1, V / V). Collect the fraction eluted with n-hexane-ethyl acet...
Embodiment 2
[0017] Example 2: In vitro anti-tumor effect of Leoleorin H
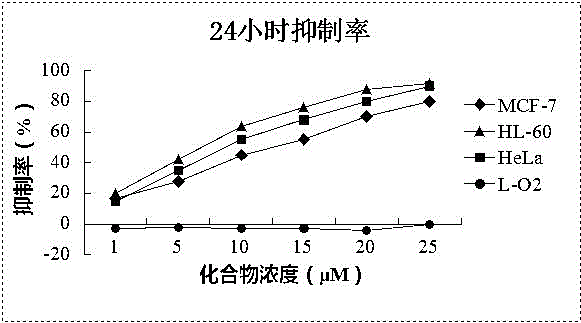
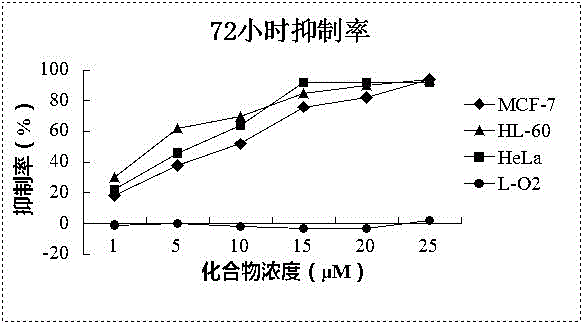
[0018] Take the cells in the logarithmic growth phase and inoculate 4~10×10 cells according to the size of the cells 3 Each was placed in a 96-well plate, and after 2 hours of growth, the supernatant was discarded, and then administered according to the following groups: Tumor cells were divided into groups without treatment and groups with treatment (concentration 1-25 μM), and each group had 5 or 6 replicate wells , cultured for 24 or 72 hours, discarded the supernatant, added 100 μl of MTT serum-free culture medium containing 0.5 mg / ml and cultured for 4 hours, added 100 μl of DMSO, placed on a micro-oscillator for 10 minutes, and then placed on a microplate reader at 570 nm Measure the OD value. Normal human cell line L-O2 was used for toxicity evaluation, and each experiment was repeated 3 times. The results are shown in Table 2 and attached figure 1 , 2 .
[0019] Table 2 IC50 results of Leoleorin H on d...
Embodiment 3
[0025] Example 3: Detection of cell apoptosis by flow cytometry
[0026] The experiment set up a negative control group (ie no drug treatment group) and a Leoleorin H treatment group (15 μM). After the cells were treated with Leoleorin H for 12 hours, they were digested with 0.1% trypsin without EDTA. After the digestion was terminated by the medium containing serum, the cells were collected into a 10ml glass centrifuge tube and centrifuged at 500rpm for 5min. The supernatant was discarded and washed with 0.01M PBS at 500rpm Centrifuge for 5 min and wash twice, then add binding liquid according to the instructions of the Annexin V / PI double staining kit and stain with flow cytometer (Becton Dickinson FACScan Flow Cytometer). (Tree Star, CA) software for data analysis. The results showed that Leoleorin H could induce apoptosis.
[0027] The apoptosis rate of the negative group without drug was 4.5±1.5% at 18 hours. The apoptosis rate of cells treated with Leoleorin H for 18 ...
PUM
 Login to View More
Login to View More Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com