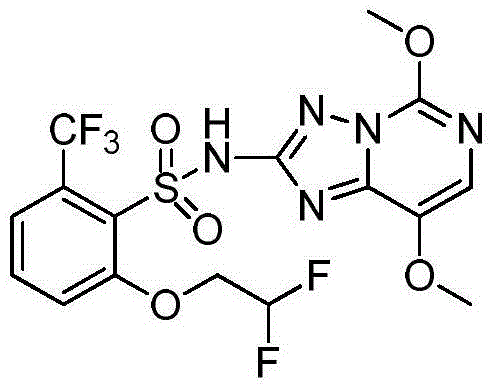
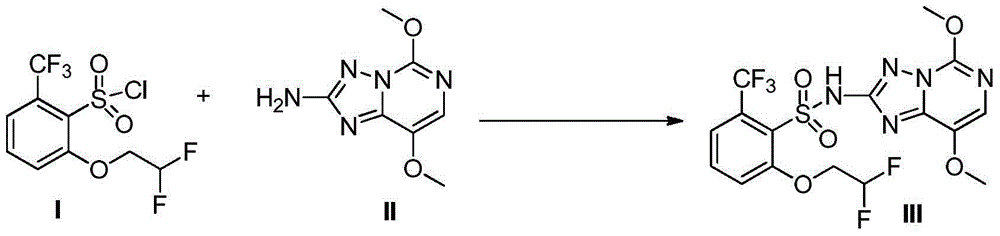
Preparation method of penoxsulam
A technology for penoxsulam and trifluoromethylbenzenesulfonyl chloride is applied in the field of preparation of penoxsulam, and can solve the problems of troublesome DMSO anhydrous treatment and recovery operations, inconvenient industrial application, low production efficiency, and the like, Achieving the effect of avoiding the use of expensive or highly toxic agents or solvents, low cost and cost saving
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
Embodiment 1
[0027]
[0028] At room temperature, add compound (II) (0.5mol) and anhydrous tetrahydrofuran (1L) into a 3L three-necked flask, add lithium diisopropylamide tetrahydrofuran solution (2.5mol / L, 300mL), stir for 10min, and dropwise add Compound (I) in tetrahydrofuran (2.5mol / L, 180mL), after the addition is complete, stir at room temperature. The reaction time is usually 30-60min until the raw material (I) or (II) is consumed. After the reaction, recover tetrahydrofuran, add 600mL of 95% ethanol to the residue, stir for 15min, filter, then stir the filter cake with 600mL of water for 10min, filter, and dry the filter cake to obtain the target product with a yield of 91%, mp: 223-224°C.
[0029] Confirmation of product structure: 1 H NMR (DMSO-d 6 ), δ: 11.88(s, 1H), 7.76(t, J=8.2Hz, 1H), 7.63(m, 3H), 6.51(t×t, J=4.2, 54.9Hz, 1H), 4.47(t×t d, J=3.8, 13.7Hz, 2H), 4.02(s, 3H), 3.84(s, 3H); ESI–MS: m / z 482 ([M-H - ]).
Embodiment 2
[0031] Using the method of Example 1, different non-nucleophilic bases or weak nucleophilic bases were used instead to synthesize the penoxsulam product. The results are shown in the following table:
[0032]
[0033]
[0034]
[0035]
[0036]
[0037]The above results show that: in the penoxsulam synthesis process method of the present invention, multiple non-nucleophilic bases or weak nucleophilic bases have good applicability to the synthetic reaction, and in (II), the base molar ratio is 1: The product yield is higher in the range of 1-1:2 process conditions, which is 87%-96%. When the consumption of alkali continued to increase (as the molar ratio was 1:3), the yield increase was very small, and the economic benefit brought by the increase in yield was less than the increased raw material cost, and the total economic benefit declined, so (II) , The molar ratio of the base is 1:1 to 1:5, preferably 1:1 to 1:2.
Embodiment 3
[0039] Using the method of Example 1, using lithium diisopropylamide as the base, changing different solvents and temperatures, the penoxsulam product was synthesized, and the results obtained are as follows:
[0040]
[0041]
[0042]
[0043]
[0044]
[0045] The above results show that: the reaction of the present invention also has good tolerance to different solvents, obtains high yields in various solvents, and has a relatively high temperature tolerance range, wherein the yield of 20 ° C to 50 ° C ideal.
PUM
 Login to View More
Login to View More Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com