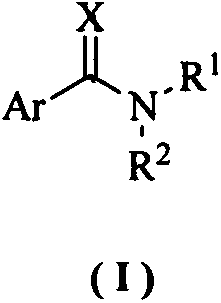
Aromatic methylamine compound and its preparation method and anti-plant virus application
A technology of anti-plant virus agent and plant virus disease, which is applied in the field of pesticides, can solve the problems of reducing the severity of symptoms, etc., and achieve the effects of low toxicity, good environmental compatibility, and excellent anti-plant virus activity
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
Embodiment 1
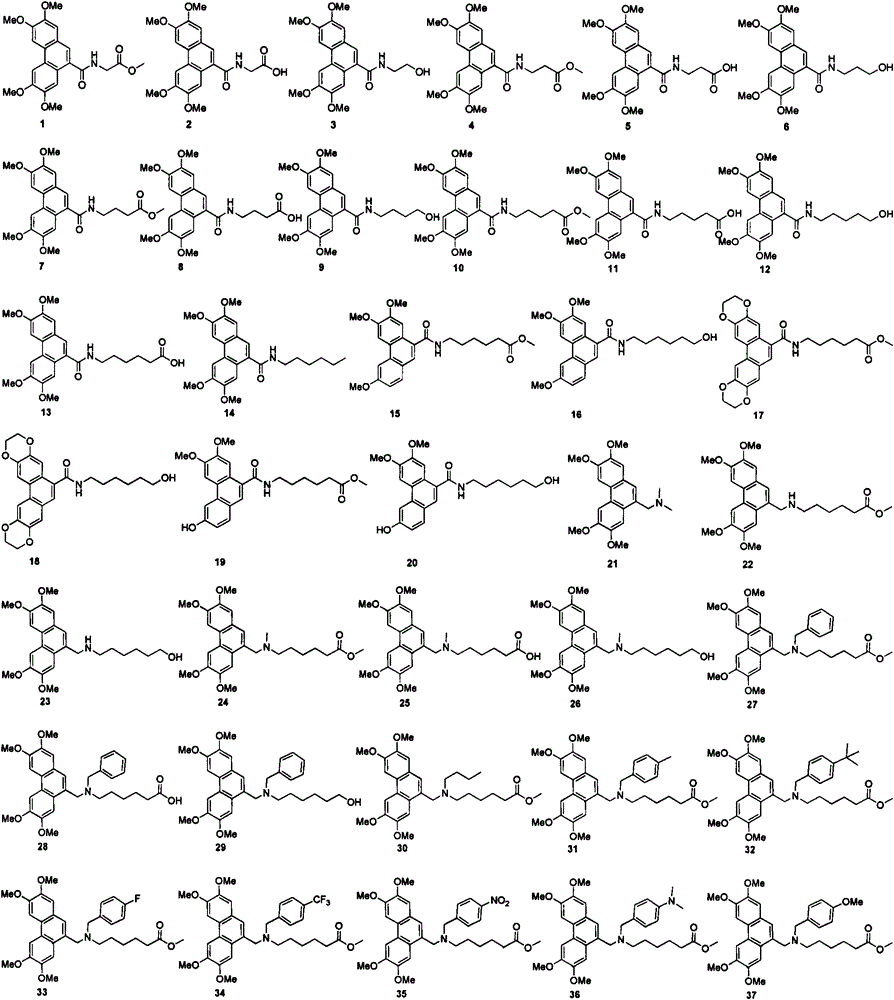
[0025] Example 1: Synthesis of arylmethylamines 1-3:
[0026]
[0027] Synthesis of 2,3,6,7-Tetramethoxy-9-phenanthrenecarbonyl chloride (67)
[0028] Add 2,3,6,7-tetramethoxy-9-phenanthrenecarboxylic acid (66) 0.80g (2.34mmol), 20mL oxalyl chloride to a 100mL single-neck bottle; add 2 drops of DMF under a water bath; then naturally rise to room temperature, The reaction was continued for another 3 h, and the oxalyl chloride was distilled off at atmospheric pressure to obtain 0.82 g of yellow solid 67; it was directly used in the next reaction without further treatment.
[0029] Synthesis of methyl 2-(N-(2,3,6,7-tetramethoxy-9-phenanthrenyl)amino)acetate (1)
[0030] Add 0.24g glycine methyl ester hydrochloride to 100mL single-neck bottle, 25mL CH 2 Cl 2 , 0.34g Et 3 N, slowly dropwise add 0.40g of 2,3,6,7-tetramethoxy-9-phenanthrenecarbonyl chloride (67) in CH under a water bath 2 Cl 2 The solution was 20 mL. After the dropwise addition, the solution was slowly raise...
Embodiment 2
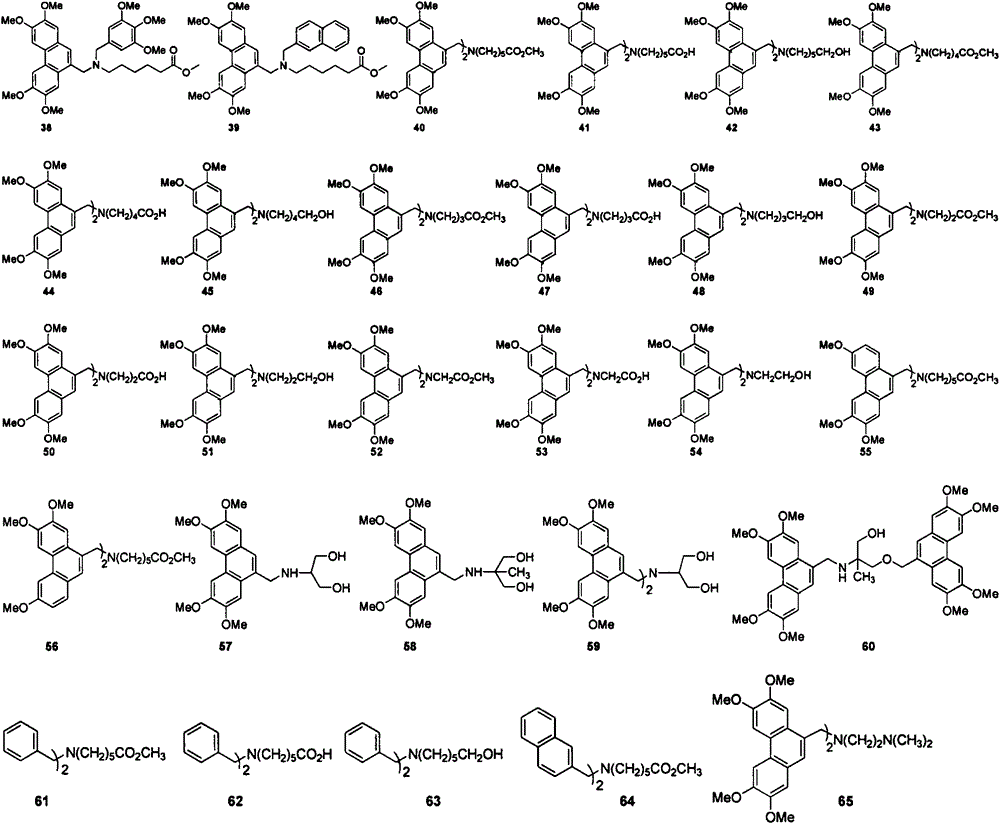
[0035] Example 2: Synthesis of arylmethylamines 22-26:
[0036]
[0037] Synthesis of 2,3,6,7-Tetramethoxy-9-phenanthrenemethanol (68)
[0038] Add 1.03g of 2,3,6,7-tetramethoxy-9-phenanthrenecarboxylic acid (66) and 20mL of THF to a 100mL single-neck bottle; add 0.28g of LiAlH in batches under ice-water bath cooling 4 , after the addition, heated to reflux for 1.5h, and then cooled to room temperature naturally. 20mL CH was added to the reaction solution 2 Cl 2 , and then slowly add 1 mol / L HCl dropwise to the ice-water bath until the white flocculent precipitate disappears; 2 Cl 2 (10 mL×3) extraction; organic phases were combined, washed once with saturated NaCl solution, anhydrous Na 2 SO 4 dry. Filtration under reduced pressure and concentration to obtain solid were recrystallized from ethyl acetate to obtain white solid, yield 95.0%, melting point: 183-185°C. 1 H NMR (400MHz, CDCl 3 )δ7.83(s, 1H), 7.78(s, 1H), 7.59(s, 1H), 7.55(s, 1H), 7.21(s, 1H), 5.13(s, 2H...
Embodiment 3
[0051] Example 3: Synthesis of arylmethylamines 4-21 and 27-65: (refer to Examples 1 and 2 for the synthesis steps, and the data are as follows)
[0052] Synthesis of methyl 3-(N-(2,3,6,7-tetramethoxy-9-phenanthrenyl)amino)propanoate (4)
[0053] White solid, yield: 90.9%, melting point: 171-172°C. 1 H NMR (400MHz, CDCl 3 )δ7.88(s, 1H), 7.78(s, 1H), 7.74(s, 1H), 7.70(s, 1H), 7.20(s, 1H), 6.75(t, J=5.6Hz, 1H), 4.13(s, 6H), 4.03(s, 6H), 3.86-3.82(m, 2H), 3.73(s, 3H), 2.77(t, J=5.6Hz, 2H); 13 C NMR (100MHz, CDCl 3 )δ173.1, 170.1, 150.2, 149.3, 148.9, 129.9, 125.5, 125.0, 124.7, 124.0, 123.1, 108.6, 106.3, 102.7, 102.5, 56.0, 56.0, 55.9, 55.9, 51.9, 35.5, 34RMS.; (m / z): calcd.forC 23 H 26 NO 7 [M+H] + 428.1704; found428.1707.
[0054] Synthesis of 3-(N-(2,3,6,7-tetramethoxy-9-phenanthrenyl)amino)propionic acid (5)
[0055] White solid, yield: 86.2%, melting point: 222-224°C. 1 H NMR (400MHz, DMSO-d 6 )δ12.27(s, 1H), 8.59(t, J=5.2Hz, 1H), 8.04(s, 1H), 8.01(s, 1H), 7.73(...
PUM
 Login to View More
Login to View More Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com