Substituted naphthyridine-2-ketone compound, preparation method, application and pharmaceutical composition
A compound and pharmacy technology, applied in uses and pharmaceutical compositions, substituted naphthyridin-2-one compounds, in the field of preparation methods, can solve the problems of large toxic and side effects, low therapeutic effect and the like
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
preparation example Construction
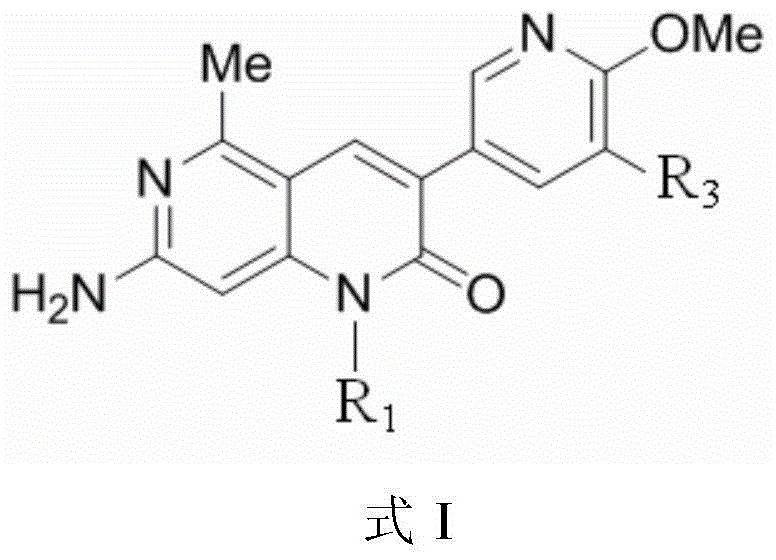
[0185] (2) The preparation method of the compound shown in formula I:
[0186] The present invention secondly provides a kind of preparation method of the compound shown in formula I, it comprises:
[0187] Make the compound shown in formula A and the compound shown in formula B react as follows under the presence of palladium catalyst, thereby obtain the compound shown in formula I:
[0188]
[0189] Among them, R 1 and R 3 as defined in claim 1; X is halogen, preferably bromine; and R 8 for or
[0190] Preferably, the palladium catalyst is bis(triphenylphosphine)palladium dichloride or [1,1'-bis(diphenylphosphino)ferrocene]palladium dichloride.
[0191] Preferably, the compound shown in formula A is prepared by the following method:
[0192] Make the compound shown in formula L and N-halogenated succinimide take place following halogenation reaction, thereby obtain the compound shown in formula A:
[0193]
[0194] More preferably, the compound shown in formu...
Embodiment 1
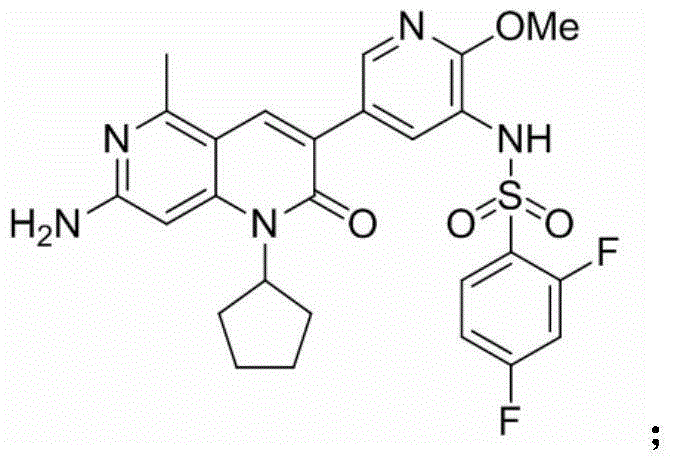
[0249] Example 1: N-(5-(7-amino-1-cyclopentyl-5-methyl-2-oxo-1,2-dihydro-1,6-naphthyridin-3-yl)-2 -Methoxypyridin-3-yl)-2,4-difluorobenzenesulfonamide (Compound 1)
[0250]
[0251] Preparation:
[0252] Step 1: Synthesis of 3-bromo-6-(2,5-methyl-1H-pyrrol-1-yl)-2-methylpyridine
[0253]
[0254] A mixture of 2-amino-5-bromo-6-methylpyridine (38.2g, 204mmol), 2,5-hexanedione (36mL, 306mmol), p-toluenesulfonic acid monohydrate (1.94g, 10.2mmol) and A three-necked flask of 380mL toluene was connected with a water separator and a condenser, and the reaction mixture was refluxed for 6h. After cooling to room temperature, the reaction mixture was diluted with ethyl acetate (500 mL), washed with water (200 mL×2) and saturated brine (200 mL×2), dried over anhydrous sodium sulfate, filtered and concentrated. The residue was purified by column chromatography (silica gel, petroleum ether / ethyl acetate=100:1, v / v) to obtain the product as a brown oil (41.4 g, yield 76%).
[025...
Embodiment 2
[0297] Example 2: N-(5-(7-amino-1-cyclopentyl-5-methyl-2-oxo-1,2-dihydro-1,6-naphthyridin-3-yl)-2 -Methoxypyridin-3-yl)-4-cyanobenzenesulfonamide (compound 2)
[0298]
[0299] Step 1: Synthesis of N-(5-bromo-2-methoxypyridin-3-yl)-4-cyanobenzenesulfonamide
[0300]
[0301] A solution of 4-cyanobenzenesulfonyl chloride (482 mg, 2.4 mmol) in dichloromethane (5 mL) was added dropwise to 3-amino-5-bromo-2-methoxypyridine (0.406 g, 2 mmol) and pyridine (237 mg, 3 mmol) in dichloromethane (5 mL), the reaction mixture was stirred at room temperature for 4 h. Water (25 mL) was added to the reaction mixture, and the resulting mixture was extracted with dichloromethane (30 mL×3). The combined organic layers were washed with water (30 mL×2) and saturated brine (30 mL×2), dried over anhydrous sodium sulfate, filtered and concentrated. The residue was separated by column chromatography (silica gel, petroleum ether / ethyl acetate=7:1, v / v) to obtain the product as a yellow solid (...
PUM
 Login to View More
Login to View More Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com