A kind of minodronic acid tablet prescription and its preparation process
A technology of minodronic acid tablet and minodronic acid monohydrate, which is applied in the field of minodronic acid tablet and its preparation, can solve problems such as unfavorable coating, easy splitting, poor tablet stability, etc., and achieve fluidity Good performance and compressibility, improved content uniformity, and easy operation
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
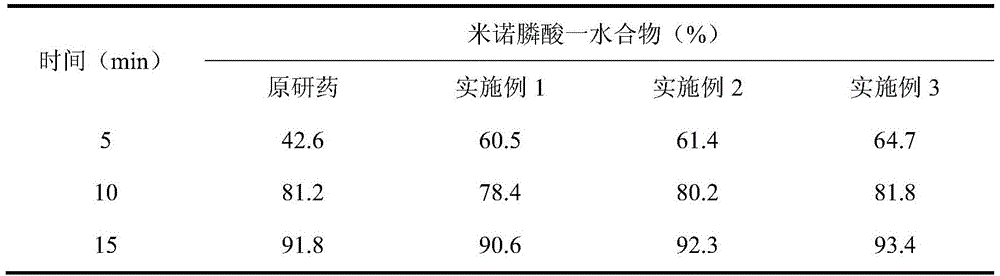
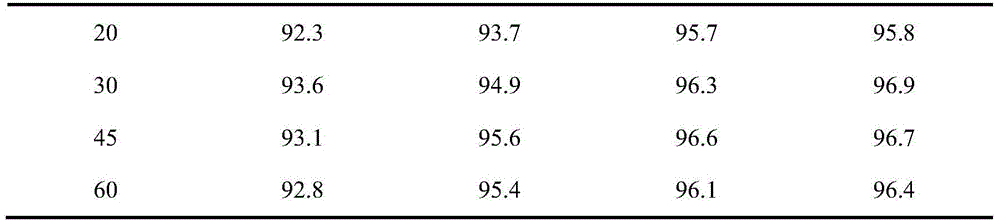
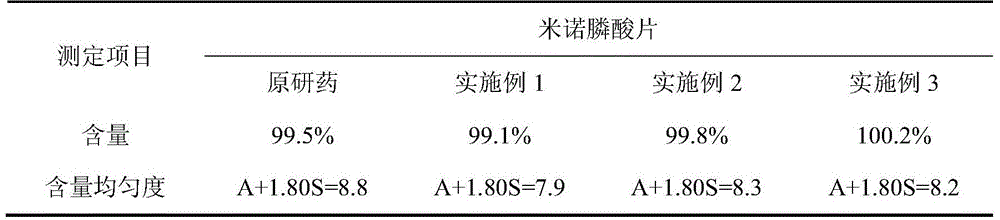
Image
Examples
Embodiment 1
[0038] Per 1000 tablets, the weight composition of each component of the tablet core is: minodronic acid monohydrate 1g, spray-dried Flowlac100 lactose 80g, corn starch 12g, polylactic acid 10g, hydroxypropyl cellulose 6g, magnesium stearate 1g,
[0039] Coating solution: use Opadry 20 g, polylactic acid 5 g dissolved in 200 g of 80% (v / v) ethanol for coating.
[0040] Preparation method, the steps are as follows:
[0041] 1) The minodronic acid monohydrate raw material is passed through a 160-mesh sieve; the spray-dried Flowlac100 lactose, corn starch, hydroxypropyl cellulose, and magnesium stearate are respectively passed through a 60-mesh sieve.
[0042] 2) Minodronic acid raw materials, direct-compressed lactose, cornstarch, polylactic acid, and hydroxypropyl cellulose were mixed in a three-dimensional motion mixer for 30 minutes, then magnesium stearate was added and mixed for 5 minutes, and the mixed material Add a high-speed rotary tablet press to compress the tablets,...
Embodiment 2
[0044] Per 1000, the weight of each component of the tablet core consists of: minodronic acid monohydrate 1g, spray-dried Flowlac100 lactose 70g, cornstarch 12g, polylactic acid 10g, hydroxypropyl cellulose 6g, magnesium stearate 1g, The above-mentioned components are coated with film coating after tableting, and the film coating solution is prepared from the following materials: Opadry 20g,
[0045] 5 g of polylactic acid was dissolved in 200 g of 80% ethanol, and mixed uniformly.
[0046] Preparation method, the steps are as follows:
[0047]1) The minodronic acid monohydrate raw material is passed through a 160-mesh sieve; the spray-dried Flowlac100 lactose, corn starch, hydroxypropyl cellulose, and magnesium stearate are respectively passed through a 60-mesh sieve.
[0048] 2) Minodronic acid raw materials, direct-compressed lactose, cornstarch, polylactic acid, and hydroxypropyl cellulose were mixed in a three-dimensional motion mixer for 30 minutes, then magnesium stear...
Embodiment 3
[0050] Every 1000 tablets, the weight of each component of the tablet core consists of: minodronic acid monohydrate 2g, spray-dried Flowlac100 lactose 59.5g, cornstarch 15g, polylactic acid 12g, hydroxypropyl cellulose 10g, magnesium stearate 1.5 g. The above-mentioned components were compressed into tablets and coated with a film coating, and the film coating liquid was prepared from the following materials: 20 g of Opadry, 5 g of polylactic acid dissolved in 200 g of 80% ethanol, and mixed uniformly.
[0051] Preparation method, the steps are as follows:
[0052] 1) The minodronic acid monohydrate raw material is passed through a 160-mesh sieve; the spray-dried Flowlac100 lactose, corn starch, hydroxypropyl cellulose, and magnesium stearate are respectively passed through a 60-mesh sieve.
[0053] 2) Minodronic acid raw materials, direct-compressed lactose, cornstarch, polylactic acid, and hydroxypropyl cellulose were mixed in a three-dimensional motion mixer for 30 minutes,...
PUM
| Property | Measurement | Unit |
|---|---|---|
| hardness | aaaaa | aaaaa |
| friability | aaaaa | aaaaa |
Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com