Montelukast sodium and levocetirizine hydrochloride gastrointestinal capsule pharmaceutical composition
A technology of levocetirizine hydrochloride and montelukast sodium hydrochloride, which is applied in the field of medicine to achieve the effects of increasing yield, reducing market risk, and solving poor stability
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
Embodiment 1
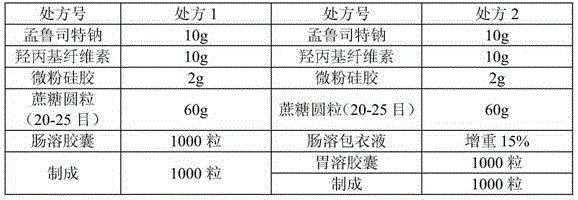
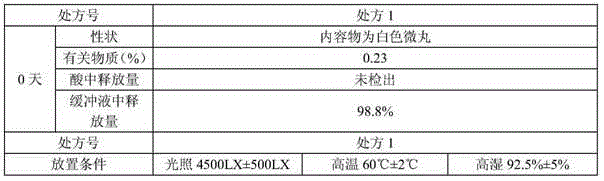
[0088] The formula for making 1000 capsules is as follows:
[0089]
[0090] Preparation Process:
[0091] 1) Take the prescribed amount of levocetirizine hydrochloride and microcrystalline cellulose, pass through an 80-mesh sieve, and use 8% gelatin solution as a binder, extrude and spheronize, prepare 20-30-mesh pellets, and dry at 50±5°C When the water content is less than 3%, levocetirizine hydrochloride gastric-soluble pellets are obtained;
[0092] 2) Mix montelukast sodium and micropowder silica gel evenly in the prescribed amount, use 8% hydroxypropyl cellulose solution as a binder, and sucrose round pellets to obtain montelukast sodium pellets. Dissolve methacrylic acid copolymer type C, talcum powder, and triethyl citrate in sodium hydroxide aqueous solution, enteric-coated to increase the weight by 15%, and dry to less than 3% moisture to obtain montelukast sodium enteric-coated pellets ;
[0093] 3) Calculate the filling amount of levocetirizine hydrochloride ...
PUM
 Login to View More
Login to View More Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com