Preparation method of vanadium oxides with different valence states, crystal forms and appearances
A technology of vanadium oxide and crystal form, which is applied in the field of preparation of vanadium oxide, can solve the problems of inability to obtain vanadium oxide, adverse environmental impact, energy consumption, etc., and achieve the effects of performance improvement, investment reduction, and uniform reaction
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
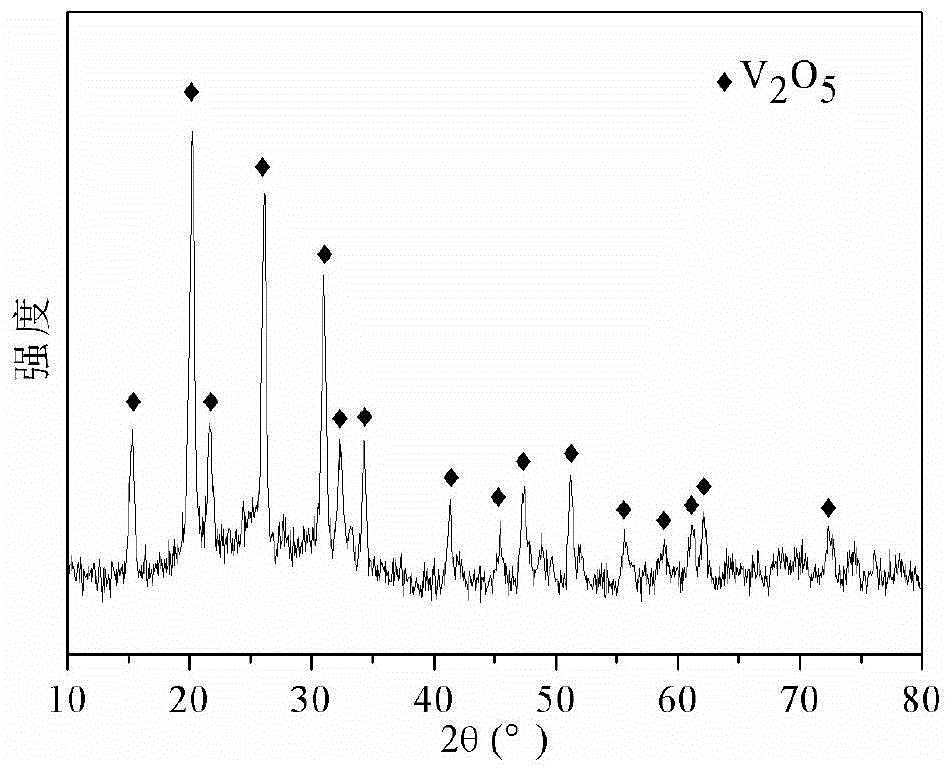
Embodiment 1
[0029] The concrete steps of preparation are:
[0030] Step 1: Firstly mix vanadium pentoxide, 28wt% hydrogen peroxide and deionized water according to the weight ratio of 0.16:3:26 to obtain a mixed solution. This mixed solution is used as a precursor solution.
[0031] Step 2, first place the precursor solution in an airtight container, and react at 180° C. for 34 minutes to obtain a reaction liquid; wherein, the airtight container is a polytetrafluoroethylene container, and the heating source is microwave. Then the reaction solution is subjected to solid-liquid separation, washing and drying in sequence; wherein, the solid-liquid separation process is centrifugation, the rotating speed is 6000r / min, and the time is 3min, and the washing process is obtained by using deionized water and ethanol to separate The solid matter is washed alternately twice, the separation of the solid matter during washing is centrifugation, and the drying process is to dry the washed solid matter...
Embodiment 2
[0033] The concrete steps of preparation are:
[0034] In step 1, vanadium pentoxide, 30wt% hydrogen peroxide and deionized water are mixed according to a weight ratio of 1:2.8:28 to obtain a mixed solution. Add polyethylene glycol to the mixed liquid; wherein, the weight ratio of polyethylene glycol to vanadium pentoxide in the mixed liquid is 0.1:1, and the polyethylene glycol is polyethylene glycol 600 to obtain a precursor solution.
[0035]Step 2, first place the precursor solution in an airtight container, and react at 200°C for 32 minutes to obtain a reaction liquid; wherein, the airtight container is a polytetrafluoroethylene container, and the heating source is microwave. Then the reaction solution is subjected to solid-liquid separation, washing and drying in sequence; wherein, the solid-liquid separation process is centrifugation, the rotating speed is 7000r / min, and the time is 2min, and the washing process is obtained by using deionized water and ethanol to separa...
Embodiment 3
[0037] The concrete steps of preparation are:
[0038] Step 1: Firstly mix vanadium pentoxide, 32wt% hydrogen peroxide and deionized water according to the weight ratio of 2:2.6:30 to obtain a mixed solution. Add polyethylene glycol to the mixed liquid; wherein, the weight ratio of polyethylene glycol to vanadium pentoxide in the mixed liquid is 0.2:2, and the polyethylene glycol is polyethylene glycol 600 to obtain a precursor solution.
[0039] Step 2, first place the precursor solution in an airtight container, and react at 220° C. for 30 minutes to obtain a reaction liquid; wherein, the airtight container is a polytetrafluoroethylene container, and the heating source is microwave. Then the reaction solution is subjected to solid-liquid separation, washing and drying in sequence; wherein, the solid-liquid separation process is centrifugation with a rotating speed of 8000r / min and a time of 1min, and the washing process is obtained by using deionized water and ethanol to sep...
PUM
| Property | Measurement | Unit |
|---|---|---|
| particle diameter | aaaaa | aaaaa |
| length | aaaaa | aaaaa |
Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com