Chiral α-amino-δ-oxopentanoate derivatives and their synthesis methods and applications
A technology of oxopentanoate and synthesis method, which is applied in the field of optically active α-amino-δ-oxopentanoate derivatives and their synthesis, and can solve the problems of difficult control of selectivity, lack of catalyst, low substrate activity, etc. problem, to achieve efficient atom economy, high selectivity, and good yield
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
Embodiment 1
[0037]
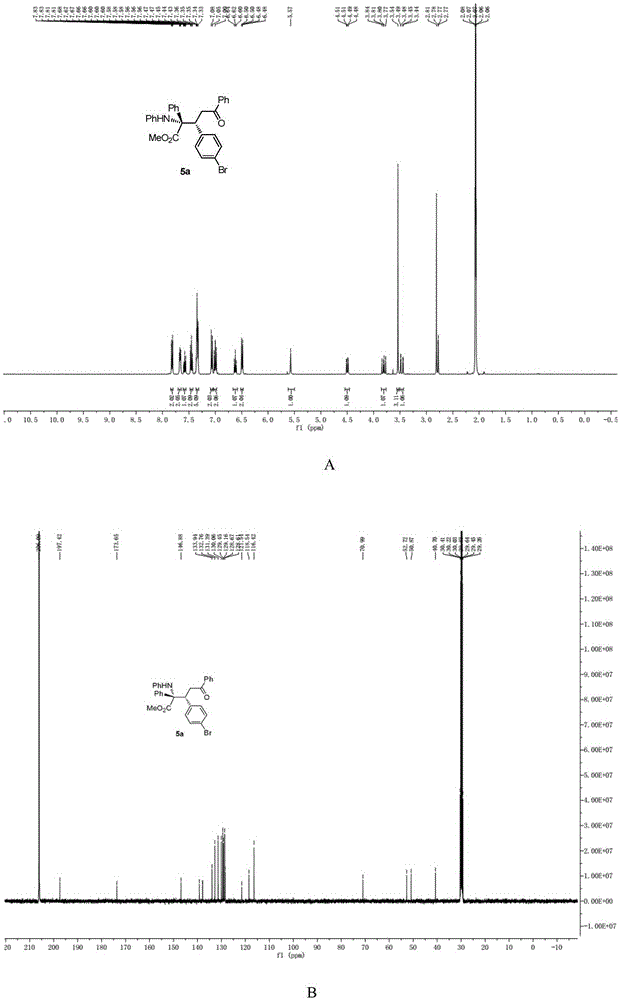
[0038] N-p-bromobenzylideneaniline (0.4mmol),[PdCl(allyl)] 2 (0.05mmol), chiral phosphoric acid and Molecular sieves (0.2g) were dissolved in chloroform (1.5mL), then methyl phenyldiazoacetate (0.8mmol) and enamine (0.8mmol) dissolved in chloroform (1.0ml) were added dropwise to In the reaction system, the reaction system was kept at -20-0°C. After the dropwise addition was completed, the reaction system was stirred for 10 minutes, and the solvent was removed under reduced pressure to obtain a crude product, the structure of which was shown in formula (2-1). The crude product was subjected to column chromatography (ethyl acetate:petroleum ether=1:80~1:40) to obtain a pure product. Yield 58%, dr=95:5, ee=60%. nuclear magnetic resonance 1 HNMR, 13 CNMR spectrum such as figure 1 as shown, 1 HNMR(400MHz,Acetone)δ7.82(dd,J=8.4,1.2Hz,2H),7.70–7.63(m,2H),7.61–7.55(m,1H),7.49–7.42(m,2H),7.37 –7.31(m,5H),7.06(d,J=8.5Hz,2H),7.00(dd,J=8.6,7.4Hz,2H),6.62(M,1H),6.49(dd,J...
Embodiment 2
[0040]
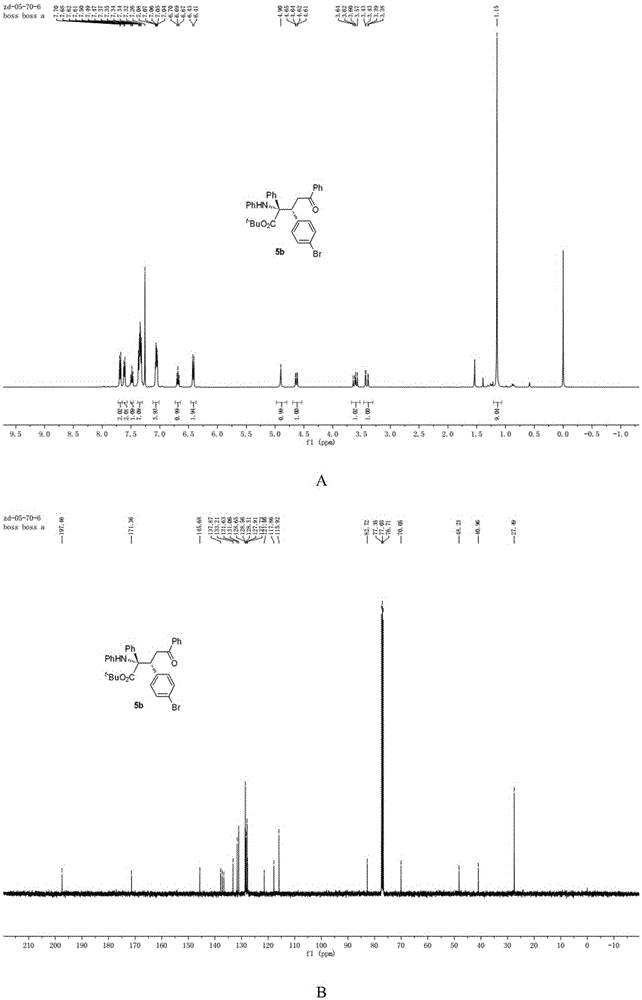
[0041] N-p-Bromobenzylideneaniline (0.4mmol),[PdCl(allyl)] 2 (0.05mmol), chiral phosphoric acid and Molecular sieves (0.2g) were dissolved in chloroform (1.5mL), then, tert-butyl phenyldiazoacetate (0.8mmol) and enamine (0.8mmol) dissolved in chloroform (1.0ml) were added dropwise in 1 hour Into the reaction system, the reaction system is at -20 ~ 0 ° C, after the dropwise addition is completed, stir for 10 minutes, and remove the solvent under reduced pressure to obtain a crude product, the structure of which is shown in formula (2-2). The crude product was subjected to column chromatography (ethyl acetate:petroleum ether=1:80~1:40) to obtain a pure product. Yield 51%, dr=95:5, ee=80%. nuclear magnetic resonance 1 HNMR, 13 CNMR spectrum such as figure 2 as shown, 1 HNMR (400MHz, CDCl 3 )δ7.71-6.67(m,2H),7.64-7.60(m,2H),7.51-7.46(m,1H),7.40–7.25(m,7H),7.15–6.87(m,6H),6.71- 6.66(m,1H),6.42(d,J=7.8Hz,1H),4.90(s,1H),4.63(dd,J=11.3,1.9Hz,1H),3.61(dd,J=17.4,11...
Embodiment 3
[0043]
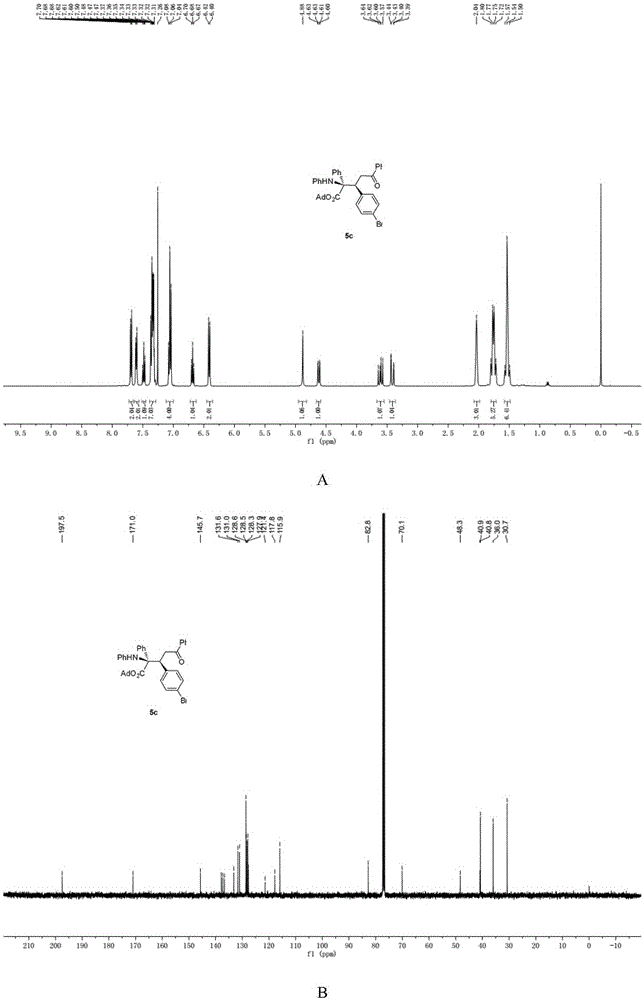
[0044] N-p-Bromobenzylideneaniline (0.4mmol),[PdCl(allyl)] 2 (0.05mmol), chiral phosphoric acid and Molecular sieves (0.2g) were dissolved in chloroform (1.5mL), then adamantyl phenyldiazoacetate (0.8mmol) and enamine (0.8mmol) dissolved in chloroform (1.0ml) were added dropwise in 1 hour Into the reaction system, the reaction system is at -20 ~ 0 ° C, after the dropwise addition is completed, stir for 10 minutes, and remove the solvent under reduced pressure to obtain a crude product, the structure of which is shown in formula (2-3). The crude product was subjected to column chromatography (ethyl acetate:petroleum ether=1:80~1:40) to obtain a pure product. Yield 69%, dr=95:5, ee=88%. nuclear magnetic resonance 1 HNMR, 13 CNMR spectrum such as image 3 as shown, 1 HNMR (400MHz, CDCl 3 )δ7.75–7.66(m,2H),7.64–7.56(m,2H),7.48(dd,J=10.5,4.3Hz,1H),7.39–7.29(m,7H),7.09-7.02(m, 1H), 6.68(t, J=7.3Hz, 1H), 6.41(d, J=7.8Hz, 1H), 4.88(s, 1H), 4.62(dd, J=11.4, 2.2Hz, ...
PUM
 Login to View More
Login to View More Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com