Method for preparing quinolone derivative
A kind of derivative and quinolone technology, applied in the field of medicinal chemistry, can solve the problems of high price and difficult availability of dimethoxybenzoic acid, and achieve the effects of short synthetic route, simple operation and easy availability of raw materials
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
preparation example Construction
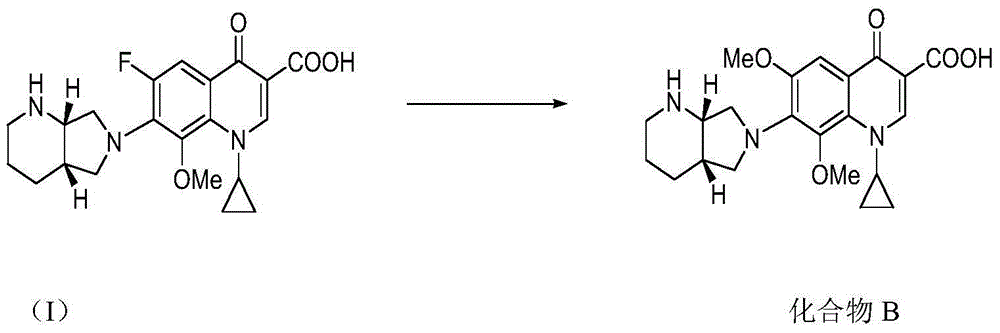
[0021] A preparation method of compound B, comprising reacting the compound represented by formula I with sodium methoxide, and after the reaction is completed, compound B is obtained through post-processing.
[0022]
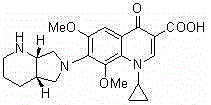
[0023] (I) Compound B
[0024] In the step, the reaction solvent is one or more of dimethylformamide, dimethyl sulfoxide, and methanol.
[0025] In said step, the reaction temperature is 80-160°C, more preferably 100°C
[0026] The present invention uses moxifloxacin and sodium methoxide as raw materials, and prepares an impurity (compound B) of moxifloxacin by substitution reaction. The synthetic route is short, the raw materials are easy to obtain, the operation is simple, and the obtained product has high purity, which is convenient to be used as a reference substance Research.
[0027] It should be understood that those skilled in the art can make various modifications and improvements to the present invention based on the contents disclosed h...
Embodiment 1
[0029] Example 1: 1-cyclopropyl-6,8-dimethoxy-1,4-dihydro-7-[(4aS,7aS)-octahydro-6H-pyrrolo[3,4-b]pyridine Preparation of -6-yl]-4-oxo-3-quinolinecarboxylic acid
[0030] Moxifloxacin (purchased from Anhui Menovo Pharmaceutical Chemical Co., Ltd.) (10 g, 0.025 mol), 100 ml of methanol and sodium methoxide (9 g, 0.167 mol) were put into an autoclave, and the temperature was raised to a reaction temperature of 145° C. for 18 hours. The reaction solution was concentrated under reduced pressure, then 70ml of water was added to adjust the pH value of the solution to 7.5, and extracted with 200ml of dichloromethane. The extracted organic layer was dried with anhydrous sodium sulfate for 1 hour. After drying, the organic layer was concentrated under reduced pressure to obtain 7g of solid. The purity detected by HPLC was 98.5%.
Embodiment 2
[0031] Example 2: 1-cyclopropyl-6,8-dimethoxy-1,4-dihydro-7-[(4aS, 7aS)-octahydro-6H-pyrrolo[3,4-b]pyridine Preparation of -6-yl]-4-oxo-3-quinolinecarboxylic acid
[0032] Moxifloxacin (10 g, 0.025 mol), 100 ml of dimethyl sulfoxide and sodium methoxide (9 g, 0.167 mol) were put into the autoclave, and the temperature was raised to 130° C. for 7 hours. The reaction solution was concentrated under reduced pressure, then 70ml of water was added to adjust the pH value of the solution to 7.5, and extracted with 200ml of dichloromethane. The extracted organic layer was dried with anhydrous sodium sulfate for 1 hour. After drying, the organic layer was concentrated under reduced pressure to obtain 8g of solid. The purity detected by HPLC was 99.2%.
PUM
 Login to View More
Login to View More Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com