Myeloperoxidase Antibody (mpo-ab) Quantitative Assay Kit
A quantitative measurement and kit technology, applied in the field of clinical immunology detection, can solve the problems of inability to distinguish non-specific recognition, expensive fluorescent microscope, and inability to carry out quantitative determination, etc., to achieve fast and reliable reaction process, high detection sensitivity and linear range , validity period and the effect of testing performance guarantee
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
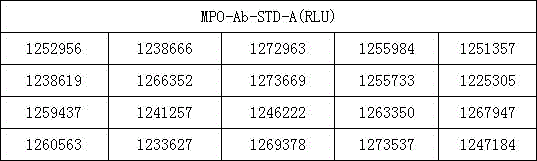
Examples
Embodiment 1
[0056] Embodiment 1, the preparation of magnetic separation reagent:
[0057] 1. The preparation process of magnetic particle buffer, taking the preparation of 1L as an example:
[0058] 1. Measure 800mL of purified water in a container, weigh 12.1g of Tris and 8.5g of NaCl into the container, stir well until completely dissolved;
[0059] 2. Weigh 5g of BSA, 50mL of newborn bovine serum, and 3000.2mL of Proclin into the container, stir well until completely dissolved;
[0060] 3. Use 4M HCl to adjust the pH, and control the pH between 7.9-8.1;
[0061] 4. Finally, dilute to 1L with purified water and store at 2-8°C until use.
[0062] 2. Preparation process of magnetic separation reagent
[0063] 1. Take magnetic particles containing carboxyl groups (COOH) active groups, the carboxyl content per gram (g) of magnetic particles (dry weight) is not less than 0.3 millimoles (mmoL), with superparamagnetic properties, and the diameter is between 0.5-2 μm;
[0064] 2. MPO antige...
Embodiment 2
[0071] Embodiment 2, the preparation of enzyme labeling reagent:
[0072] 1. The preparation process of enzyme labeling reagent diluent, taking the preparation of 1L as an example:
[0073] 1. Measure 800mL of purified water in a container, weigh 6.06g of Hepes and 8.5g of NaCl into the container, and stir until completely dissolved;
[0074] 2. Weigh BSA5g, Zncl 2 0.1g, Proclin-3000.2mL and MgCl 2 0.1g in the container, fully stirred until completely dissolved;
[0075] 3. Use 4M HCl to adjust the pH, and control the pH between 7.5-8.0;
[0076] 4. Finally, dilute to 1L with purified water and store at 2-8°C until use.
[0077] 2. Coupling of alkaline phosphatase (ALP) to MPO antibody
[0078] 1. MPO antibody, purchased from Beijing Jiufeng Runda Biotechnology Co., Ltd., with a purity of more than 95%;
[0079] 2. The purity of alkaline phosphatase should exceed 95%, the specific activity should exceed 1000U / mg, and the concentration should exceed 5mg / mL;
[0080] 3. T...
Embodiment 3
[0084] Embodiment 3, the preparation of calibrator:
[0085] one, Preparation of calibrator dilutions:
[0086] 1. Measure 600mL of purified water in a container, weigh 200mL of newborn bovine serum and add it to the container, stir well and mix evenly;
[0087] 2. Weigh 3.4g of dipotassium hydrogen phosphate, 0.36g of potassium dihydrogen phosphate, and 3000.02mL of Proclin into the container, stir well until completely dissolved;
[0088] 3. Use 4M HCl to adjust the pH, and control the pH between 7.0-7.5;
[0089] 4. Finally, dilute to 1L with purified water and store at 2-8°C until use.
[0090] 2. Preparation of calibrator:
[0091] 1. Prepare the MPO antibody at 0, 5, 20, 50, 150, and 300 U / mL with the calibrator diluent, a total of 6 concentration points.
PUM
| Property | Measurement | Unit |
|---|---|---|
| diameter | aaaaa | aaaaa |
| concentration | aaaaa | aaaaa |
Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com