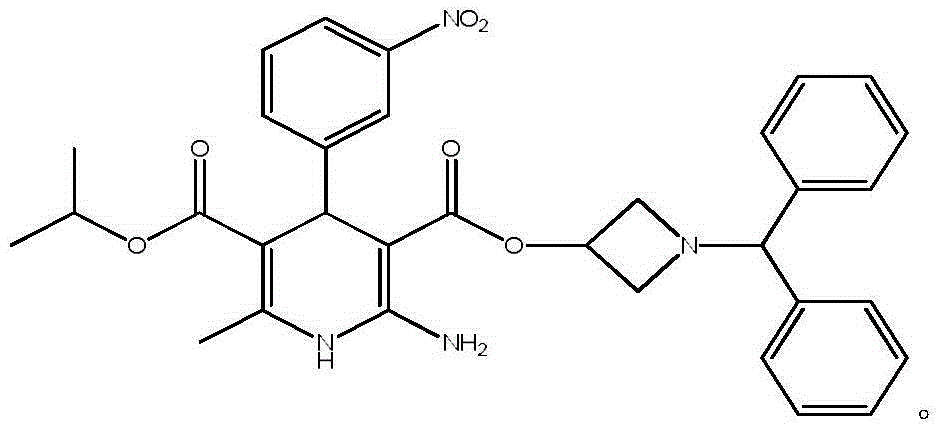
Pharmaceutical composition of azelnidipine
A technology of azendipine and its composition, which is applied in the field of pharmaceutical compositions of dihydropyridine calcium ion antagonists, and can solve the problems of azendipine’s stability, influence on drug quality and curative effect, slow degradation, etc.
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
Embodiment 1
[0044] Tablet 1:
[0045]
[0046]
[0047] Preparation process: Mix the prescribed amount of Azhedipine with a part of direct-pressed mannitol and pass through a 60-mesh sieve, then add the remaining prescription mannitol, low-substituted hydroxypropyl cellulose, meglumine, magnesium oxide, and calcium carbonate and mix through a 60-mesh sieve , and finally add the prescribed amount of magnesium stearate and mix well. Then punch the tablet with a shallow circular arc with a diameter of 7.5 mm to obtain the Azhedi flat tablet.
[0048] The dissolution rate of this preparation was measured in 75prm, 0.1M hydrochloric acid for 45min, and the dissolution rate was 99.4%.
Embodiment 2
[0050] Capsule 1:
[0051] According to the prescription of the above-mentioned embodiment 1, the mixed powder obtained in the above example is packed into 3# capsules with the powder direct filling process. The dissolution rate of this preparation was measured in 75prm, 0.1M hydrochloric acid for 45min, and the dissolution rate was 99.6%.
Embodiment 3
[0053] tablet 2
[0054]
[0055] Preparation process: Mix the prescribed amount of Azedipine with mannitol, microcrystalline cellulose, croscarmellose sodium, magnesium oxide, calcium carbonate, and micropowder silica gel in a high-efficiency wet mixer, and then add the dissolved prescription Stir the 5% HPMC aqueous solution of meglumine to make soft material, sieve with 20-mesh stainless steel screen to make wet granules; put the wet granules in a ventilated and constant temperature drying oven at 50°C for drying, and sieve the granules with 20-mesh sieve after drying , while adding magnesium stearate and mixing evenly to obtain dry granules of azedipine. Then punch the tablet with a shallow circular arc with a diameter of 7.5 mm to obtain the Azhedi flat tablet.
[0056] The dissolution of the preparation was measured in 75prm, 0.1M hydrochloric acid for 45min, and the dissolution rate was 99.5%.
PUM
| Property | Measurement | Unit |
|---|---|---|
| Diameter | aaaaa | aaaaa |
Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com