Non-vanadium denitration catalyst, preparation method and applications thereof
A denitration catalyst and catalyst technology, which are applied in chemical instruments and methods, physical/chemical process catalysts, separation methods, etc., can solve the problems of low denitration performance of catalysts, large temperature fluctuation range, and high toxicity of precursors, and achieve widening of the active temperature. window, improve the safety of use, improve the effect of denitration performance
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
Embodiment 1
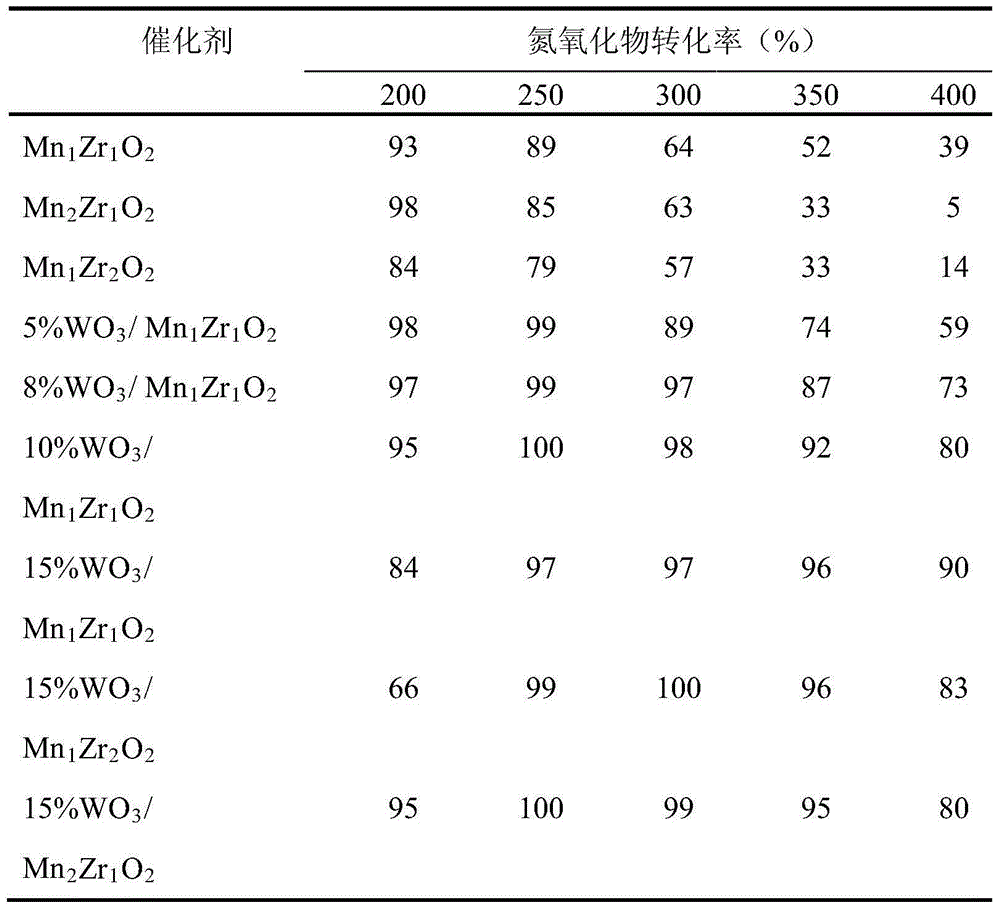
[0016] Example 1: 5% WO 3 / Mn 1 Zr 1 o 2 Preparation of composite catalyst
[0017] a) Take 40ml of 0.5mol / L zirconium nitrate solution and 20ml of 1.0mol / L manganese nitrate solution respectively, stir and mix in a water bath at 50°C for 60 minutes to obtain a mixed solution;
[0018] b) Add ammonia water to the mixed solution obtained in step a) under constant stirring until the pH value is 10, transfer the obtained mixed solution to a hydrothermal reaction kettle, perform a hydrothermal reaction at 120° C. for 4 hours, and then lower it to room temperature;
[0019] c) Suction filter the reaction solution obtained in step b), wash, dry at 120°C for 24 hours, and then bake in a muffle furnace at 500°C for 12 hours to obtain a manganese-zirconium composite oxide;
[0020] d) Take 2.94ml of 0.02mol / L ammonium metatungstate solution, stir in a 30°C water bath to obtain a mixed solution; take 1.81g of the powder obtained in c) and add it to the mixed solution, and stir for 2...
Embodiment 2
[0022] Example 2: 8% WO 3 / Mn 1 Zr 1 o 2 Preparation of composite catalyst
[0023] a) Take 40ml of 1.0mol / L zirconium nitrate solution and 20ml of 2.0mol / L manganese nitrate solution respectively, stir and mix in a water bath at 40°C for 90 minutes to obtain a mixed solution;
[0024] b) Add ammonia water to the mixed solution obtained in step a) under constant stirring until the pH value is 10, transfer the obtained mixed solution to a hydrothermal reaction kettle, perform a hydrothermal reaction at 120° C. for 8 hours, and then lower it to room temperature;
[0025] c) Suction filter the reaction solution obtained in step b), wash, dry at 120°C for 24 hours, and then bake in a muffle furnace at 500°C for 12 hours to obtain a manganese-zirconium composite oxide;
[0026] d) Take 4.51ml of 0.04mol / L ammonium metatungstate solution and stir in a 30°C water bath to obtain a mixed solution; take 3.37g of the powder obtained in c) and add it to the mixed solution, and stir fo...
Embodiment 3
[0028] Example 3: 10% WO 3 / Mn 1 Zr 1 o 2 Preparation of composite catalyst
[0029] a) Take 30ml of 1.0mol / L zirconium nitrate solution and 15ml of 2.0mol / L manganese nitrate solution respectively, stir and mix in a water bath at 30°C for 30 minutes to obtain a mixed solution;
[0030] b) adding ammonia water to the mixed solution obtained in step a) under constant stirring until the pH value is 10, transferring the obtained mixed solution to a hydrothermal reaction kettle, performing a hydrothermal reaction at 120° C. for 12 hours, and then cooling down to room temperature;
[0031] c) Suction filter the reaction solution obtained in step b), wash, dry at 120°C for 12 hours, and then bake in a muffle furnace at 500°C for 4 hours to obtain a manganese-zirconium composite oxide;
[0032] d) Take 3.46ml of 0.05mol / L ammonium metatungstate solution, stir in a 30°C water bath to obtain a mixed solution; take 2.53g of the powder obtained in c) and add it to the mixed solution,...
PUM
 Login to View More
Login to View More Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com