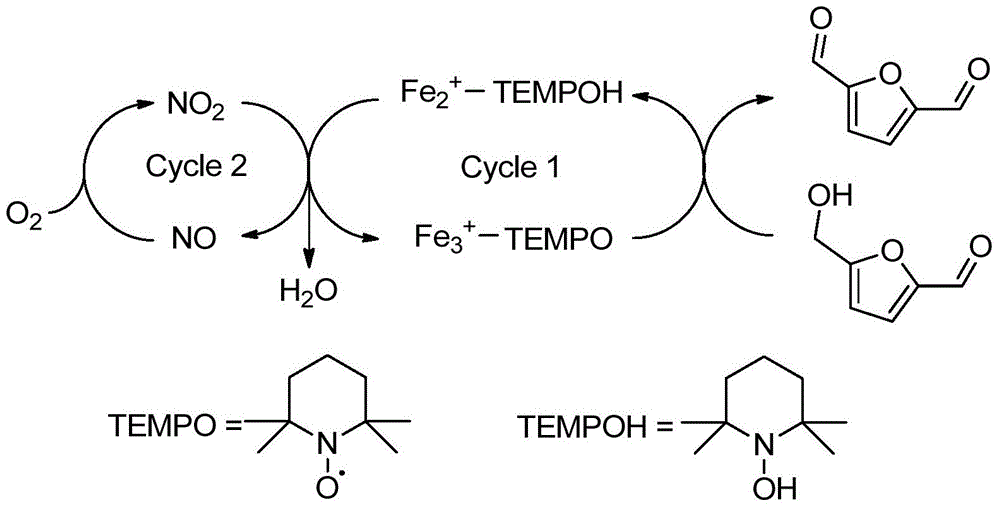
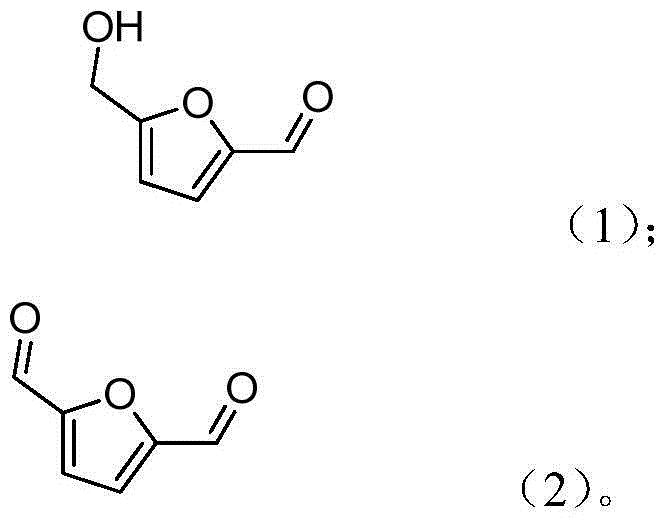
Method for preparing 2,5-diformylfuran
A technology of furandiformaldehyde and hydroxymethyl furfural, applied in directions such as organic chemistry, can solve problems such as difficulty in product separation, and achieve the effects of low economic and environmental cost, easy reuse, and low amount of by-products
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
Embodiment 1
[0031] In this example, 2,5-furandicarbaldehyde (2,5-DFF) was prepared according to the following steps:
[0032] Dissolve 63 mg of 5-HMF (total 0.5 mmol) in 2 mL of dichloroethane, then add 10.1 mg of Fe(NO 3 ) 3 9H 2 O (a total of 0.025mmol, accounting for 5% of the amount of 5-HMF substances), 7.8mg of TEMPO (a total of 0.025mmol, accounting for 5% of the amount of 5-HMF substances) and 1.5mg NaCl (a total of 0.025mol, accounting for 5- 5% of the amount of the HMF substance) to obtain a mixed liquid of the raw material group, and stirred and reacted in the air at room temperature and pressure for 4 hours to obtain a reaction mixture.
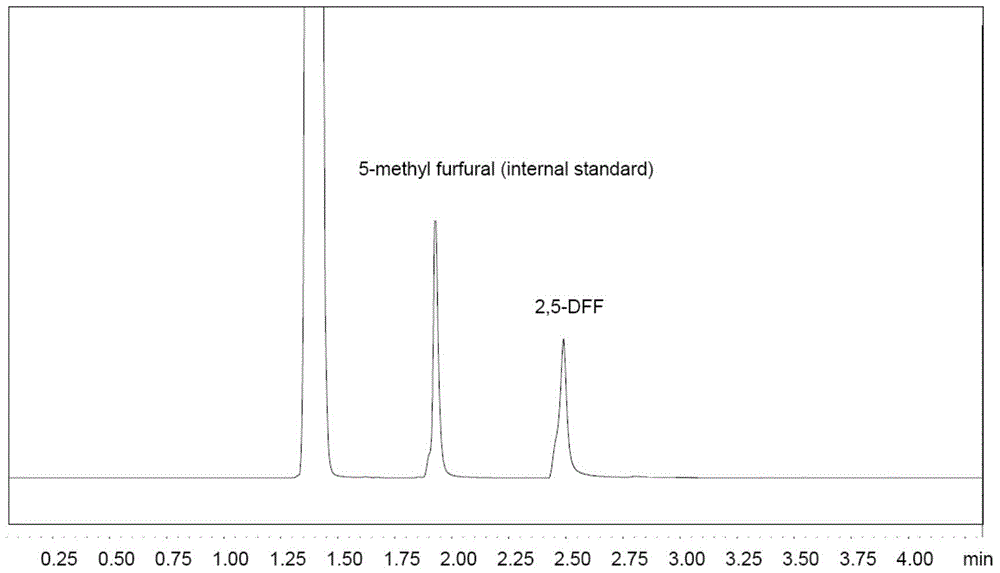
[0033] The GC detection result of reaction mixture is as follows figure 2 shown, from figure 2 It can be seen that the product 2,5-furandicarbaldehyde (internal standard: 5-methylfurfural) was successfully obtained in this implementation, and the yield of 2,5-DFF in this example was 92%.
[0034] Conditions for GC detection:
[0035] ...
Embodiment 2
[0039]This embodiment prepares the raw material group as shown in Table 1, and in addition to the raw materials covered by Table 1, also contains 0.5mmol of 5-HMF and 2mL of organic solvent dichloromethane, the preparation method is: 0.5mmol 5-HMF is added to In 2mL of dichloromethane, add TEMPO, catalyst activator (Fe(NO 3 ) 3 9H 2 O, Cu(NO 3 ) 2 ·3H 2 O, AgNO 3 , Pd(NO 3 ) 3 ·3H 2 O or FeCl 3 ) and additives (NaCl) to form a raw material mixture, stirring the raw material mixture at room temperature and pressure for 4 hours, so that 5-hydroxymethylfurfural can The oxidation reaction is carried out to obtain the product 2,5-furandicarbaldehyde. The yields of the products obtained are shown in Table 1.
[0040] Table 1 The table of raw materials used in embodiment 2
[0041]
[0042] As can be seen from the yield in the table, no target compound can be obtained without adding TEMPO (Example 2) or without adding iron nitrate nonahydrate (Example 3). Adding a sma...
Embodiment 3
[0044] This embodiment prepares the raw material group as shown in Table 2, and in addition to the raw materials covered by Table 2, it also contains 0.5mmol of 5-HMF and 2mL of organic solvent dichloromethane. The preparation method is: 0.5mmol 5-HMF is added to In 2mL of dichloromethane, add TEMPO, catalyst activator (Fe(NO 3 ) 3 9H 2 O) and additives (NaBr, KCl, NaF or NaCl) to form a raw material mixture, and stir the raw material mixture for 4 hours at room temperature and normal pressure, so that 5-hydroxymethylfurfural is catalyzed by the piperidine nitrogen oxide catalyst and the catalyst activator. 1. Carry out the oxidation reaction under the oxidation of air to obtain the product 2,5-furandicarbaldehyde. The yields of the products obtained are shown in Table 2.
[0045] Table 2 The table of raw materials used in embodiment 3
[0046]
[0047] It can be seen from the yield in the table that adding a small amount of alkali metal halide can affect the yield of 2...
PUM
 Login to View More
Login to View More Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com