Use of the cell-penetrating peptide lta2 from the lt subunit as an intracellular drug delivery vehicle
A drug and cell technology, applied in the direction of drug combination, medical preparations with non-active ingredients, medical preparations containing active ingredients, etc., to achieve the effect of high membrane penetration efficiency, less unsafe factors, and less quantity
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
Embodiment 1
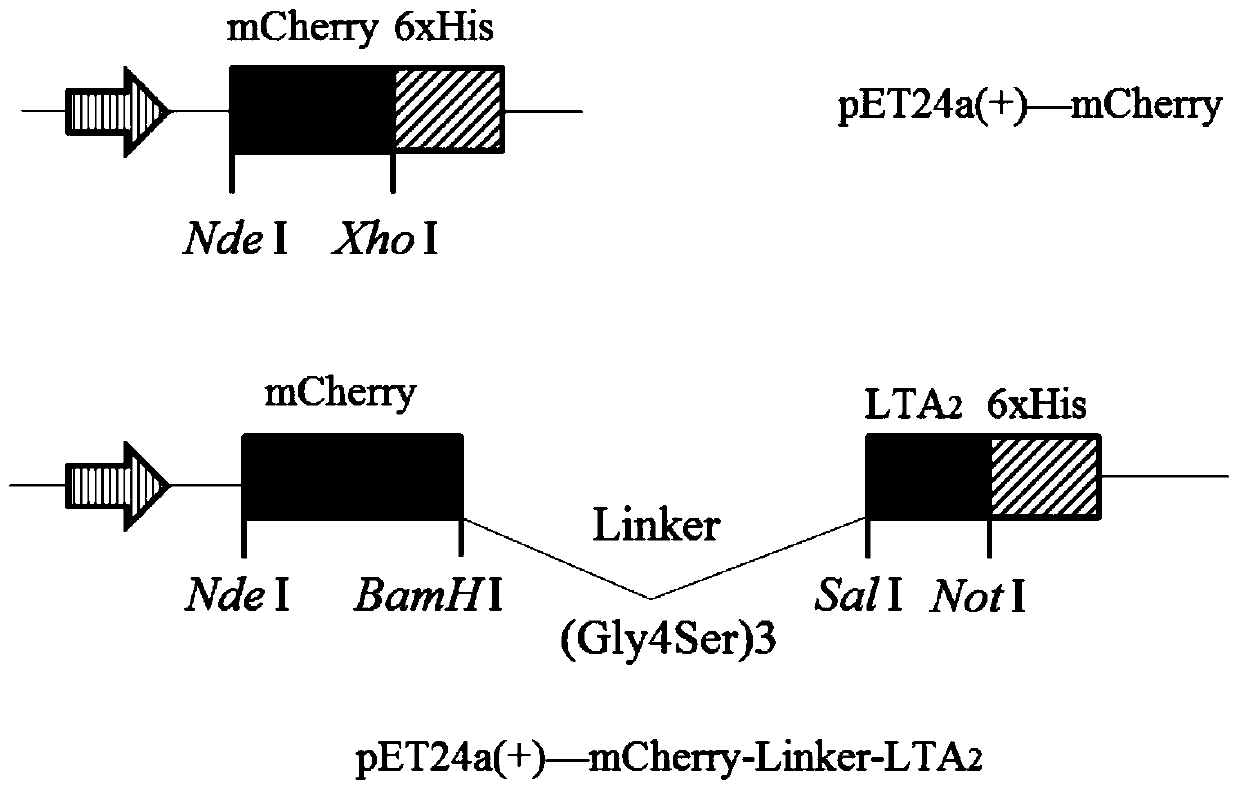
[0029] Example 1. pET24a-mCherry and pET24a-mCherry-linker-LTA 2 Construction of recombinant plasmids
[0030] Reagents and Kits: DNA Polymerase Pyrobest TM , restriction endonucleases (Nde I, XhoI, BamH I, Sal I, Not I), DNA ligation kit DNA Ligation Kit Ver.2.1, agarose gel extraction kit TaKaRaMiniBEST Agarose Gel DNA Extraction Kit Ver.4.0, plasmid The extraction kit TaKaRaMiniBEST Plasmid Purification Kit Ver.4.0 was purchased from Treasure Bioengineering Co., Ltd. (Dalian). Tryptone and yeast extract were purchased from Oxoid Company (UK). Kanamycin sulfate was purchased from Sigma-Aldrich (USA). Other biochemical reagents belong to domestic conventional analytical reagents.
[0031] Plasmids and strains: Plasmid pET-24a(+) (Novagen, USA), E.coliDH5α (Invirogen, USA) were used as plasmid amplification strains.
[0032] Reagent preparation:
[0033] LB liquid medium: Weigh 10 g of tryptone, 5 g of yeast extract, and 10 g of sodium chloride, dilute to 1 L with deioni...
Embodiment 2
[0090] Example 2. mCherry protein and mCherry-linker-LTA 2 Protein expression and purification
[0091] Reagents and kits: Isopropyl B-D-I-Thiogalactopyranoside (IPTG), kanamycin sulfate were purchased from Sigma-Aldrich (USA), Ni-NTA affinity column and filler Purchased from Biorad Company (USA), protein molecular weight standard product Premixed Protein Marker (Low), protein loading buffer 4×Protein SDS PAGE Loading Buffer, purchased from Bao Biological Engineering Co., Ltd. (Dalian). Bradford protein quantification kit was purchased from Tiangen Biochemical Technology Co., Ltd. (Beijing). Other biochemical reagents belong to domestic conventional analytical reagents.
[0092] Strains and plasmids: E. coli BL21(DE3) (Invirogen, USA) was used as a plasmid expression strain. Recombinant plasmid pET24a-mCherry, pET24a-mCherry-linker-LTA 2 Constructed for the above-mentioned stages of the present invention.
[0093] Reagent preparation:
[0094] 5× protein electrophoresis ...
Embodiment 3
[0109] Example 3.LTA 2 Transmembrane and translocation of proteins to cells
[0110] Reagents and cell lines: McCoy's 5A medium, RPMI-1640 medium, fetal bovine serum (FBS), trypsin were purchased from Gibco (USA), penicillin and streptomycin mixed solution was purchased from Suleibao (Beijing). Other biochemical reagents belong to domestic conventional analytical reagents. Hct-116 cell line, Bcap-37 cell line and A549 cell line were purchased from the Cell Bank of the Type Culture Collection Committee of the Chinese Academy of Sciences (Shanghai).
[0111] Reagent configuration:
[0112] Preparation of 10% fetal bovine serum medium: Measure 180ml of McCoy's 5A medium or 180ml of RPMI-1640 medium, add 20ml of fetal bovine serum, then add 2ml of 100× penicillin and streptomycin mixture, mix well and place at 4°C save.
[0113] Preparation of phosphate buffer: weigh 8NaCl, 1.42gNa 2 HPO 4 , 0.24g KHPO 4 , 0.2g KCl, after dissolving a small amount of ultrapure water, add ul...
PUM
| Property | Measurement | Unit |
|---|---|---|
| purity | aaaaa | aaaaa |
Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com