Method for preparing reactive type halogen-free flame retardant for coating
A reactive flame retardant technology, applied in fire-resistant coatings, chemical instruments and methods, and compounds of Group 5/15 elements of the periodic table, etc., can solve the problems of reducing coating adhesion and weather resistance, and achieve flame retardant performance Good, simple process, and easy-to-obtain raw materials
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
Embodiment 1
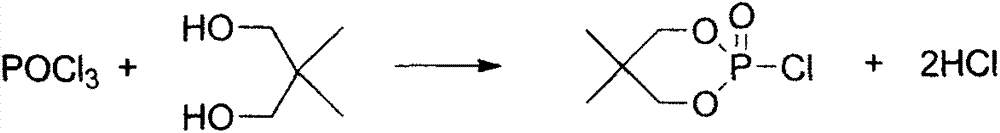
[0021] (1) In a reactor equipped with a thermometer, dropping funnel, nitrogen inlet pipe, and reflux condenser (the tail end is connected to an anhydrous calcium chloride drying tube and a hydrogen chloride absorption bottle), add 0.1mol neopentyl dichloride Alcohol and 300ml chloroform, stirred and heated to the reflux temperature, then added dropwise 0.1mol phosphorus oxychloride, reacted at the reflux temperature after the dropwise addition until no HCl gas was released at the outlet tube, and then removed the solvent to obtain a white solid 2-oxo- 2-Chloro-5,5-dimethyl-1,3,2-dioxaphosphorinane.
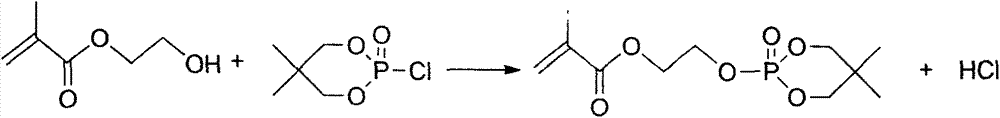
[0022] (2) Add 0.1mol 2-oxo-2-chloro-5,5-dimethyl-1,3,2-dioxaphosphorinane and 3ml of phosphine in the reactor as in step (1) After the acid agent and 300ml dimethyl sulfoxide were stirred and dissolved, 0.1mol hydroxyethyl methacrylate was added, and heated to reflux temperature until almost no HCl gas was released. The dispersion medium was distilled off under reduced pressure...
Embodiment 2
[0024] (1) In the second reactor equipped with a thermometer, a dropping funnel, a nitrogen inlet pipe, and a reflux condenser (the tail section is connected to an anhydrous calcium chloride drying tube and a hydrogen chloride absorption bottle), add 0.15mol neopentyl Glycol and 300ml chloroform, stirred and heated to the reflux temperature, then added dropwise 0.1mol phosphorus oxychloride, reacted at the reflux temperature after the dropwise addition until no HCl gas was released at the outlet tube, and then removed the solvent to obtain a white solid 2-oxo -2-Chloro-5,5-dimethyl-1,3,2-dioxaphosphorinane.
[0025] (2) Add 0.1mol 2-oxo-2-chloro-5,5-dimethyl-1,3,2-dioxaphosphorinane and 3ml of phosphine in the reactor as in step (1) After the acid agent and 300ml dimethyl sulfoxide were stirred and dissolved, 0.1mol hydroxyethyl methacrylate was added, and heated to reflux temperature until almost no HCl gas was released. The dispersion medium was distilled off under reduced ...
Embodiment 3
[0027] (1) In a reactor equipped with a thermometer, dropping funnel, nitrogen inlet pipe, and reflux condenser (the tail end is connected to an anhydrous calcium chloride drying tube and a hydrogen chloride absorption bottle), add 0.1mol neopentyl dichloride Alcohol and 300ml chloroform, stirred and heated to the reflux temperature, then added dropwise 0.1mol phosphorus oxychloride, reacted at the reflux temperature after the dropwise addition until no HCl gas was released at the outlet tube, and then removed the solvent to obtain a white solid 2-oxo- 2-Chloro-5,5-dimethyl-1,3,2-dioxaphosphorinane.
[0028] (2) Add 0.1mol 2-oxo-2-chloro-5,5-dimethyl-1,3,2-dioxaphosphorinane and 3ml of phosphine in the reactor as in step (1) After the acid agent and 300ml dimethyl sulfoxide were stirred and dissolved, 0.15mol hydroxyethyl methacrylate was added, and heated to reflux temperature until almost no HCl gas was released. The dispersion medium was distilled off under reduced pressur...
PUM
 Login to View More
Login to View More Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com