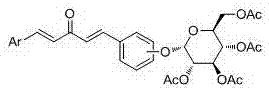
Glucoside-1,4-pentadiene-3-ketone compounds containing acetoxyl groups as well as preparation method and use of glucoside-1,4-pentadiene-3-ketone compounds
A kind of technology of acetoxyglucose and ketone compounds, applied in the field of chemistry
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
Embodiment 1
[0087] Embodiment one: compound (1 E ,4 E )-1-(4-(2,3,4,6-four- O -Acetyl- β- Synthesis of D-glucopyranoside)oxyphenyl)-5-phenyl-1,4-pentadien-3-one:
[0088] (1) Intermediate ( E )-4-(4-hydroxyphenyl)-3-buten-2-one: Add 16.4 mmol p-hydroxybenzaldehyde and 164 mmol acetone to a 50 mL three-necked flask, slowly add 18 mL of 1 mol / L sodium hydroxide solution, stirred at room temperature for 14 hours, then stopped the reaction, poured the reaction solution into 20mL of ice water, adjusted the pH to 5~6 with dilute hydrochloric acid, a large amount of yellow solids precipitated, and then stirred for 0.5 hours , suction filtered, washed with water, dried, and then recrystallized with ethyl acetate;
[0089] (2) Intermediate (1 E ,4 E )-1-phenyl-5-(4-hydroxyphenyl)-1,4-pentadien-3-one synthesis: add 5 mmol ( E )-4-(4-hydroxyphenyl)-3-buten-2-one), 5.5 mmol benzaldehyde, 15 mL absolute ethanol, slowly drop 0.5 g sodium hydroxide solution in 2 mL at room temperature, and stir ...
Embodiment 2
[0091] Embodiment two: (1 E ,4 E )-1-(2-(2,3,4,6-four- O -Acetyl- β- Synthesis of D-glucopyranoside)oxyphenyl)-5-phenyl-1,4-pentadien-3-one:
[0092] (1) Intermediate ( E )-4-(2-hydroxyphenyl)-3-buten-2-one synthesis: the synthesis steps and process conditions are the same as in Example 1 (1), the difference is that o-hydroxybenzaldehyde is a raw material;
[0093] (2) Intermediate (1 E ,4 E )-1-phenyl-5-(2-hydroxyphenyl)-1,4-pentadien-3-one synthesis: the synthesis steps and process conditions are the same as in Example 1 (2);
[0094] (3) Target product (1 E ,4 E )-1-(2-(2,3,4,6-four- O -Acetyl- β- Synthesis of D-glucopyranoside)phenyl)-5-phenyl-1,4-pentadien-3-one: the synthesis steps and process conditions are the same as in Example 1 (3).
Embodiment 3
[0095] Embodiment three: compound (1 E ,4 E )-1-(4-(2,3,4,6-four- O -Acetyl- β- Synthesis of D-glucopyranoside)phenyl)-5-(4-fluorophenyl)-1,4-pentadien-3-one:
[0096] (1) Intermediate ( E )-4-(4-hydroxyphenyl)-3-buten-2-one synthesis: the synthesis steps and process conditions are the same as Example 1 (1);
[0097] (2) Intermediate (1 E ,4 E )-1-(4-fluorophenyl)-5-(4-hydroxyphenyl)-1,4-pentadien-3-one synthesis: the synthesis steps and process conditions are the same as in Example 1 (2); The difference is that p-fluorobenzaldehyde is used as raw material;
[0098] (3) Target product (1 E ,4 E )-1-(4-(2,3,4,6-four- O -Acetyl- β- Synthesis of D-glucopyranoside)phenyl)-5-(4-fluorophenyl)-1,4-pentadien-3-one: the synthesis steps and process conditions are the same as in Example 1 (3).
PUM
 Login to View More
Login to View More Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com