A method for in vitro differentiation of induced pluripotent stem cells into ventricular myocytes
A technology of pluripotent stem cells and ventricular myocytes, which is applied in the field of pluripotent stem cell differentiation and cell signal transduction, can solve the problems of unidentified ventricular muscle, inability to achieve directional differentiation of different cardiomyocytes, and low efficiency of cardiomyocyte differentiation.
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
Embodiment 1B
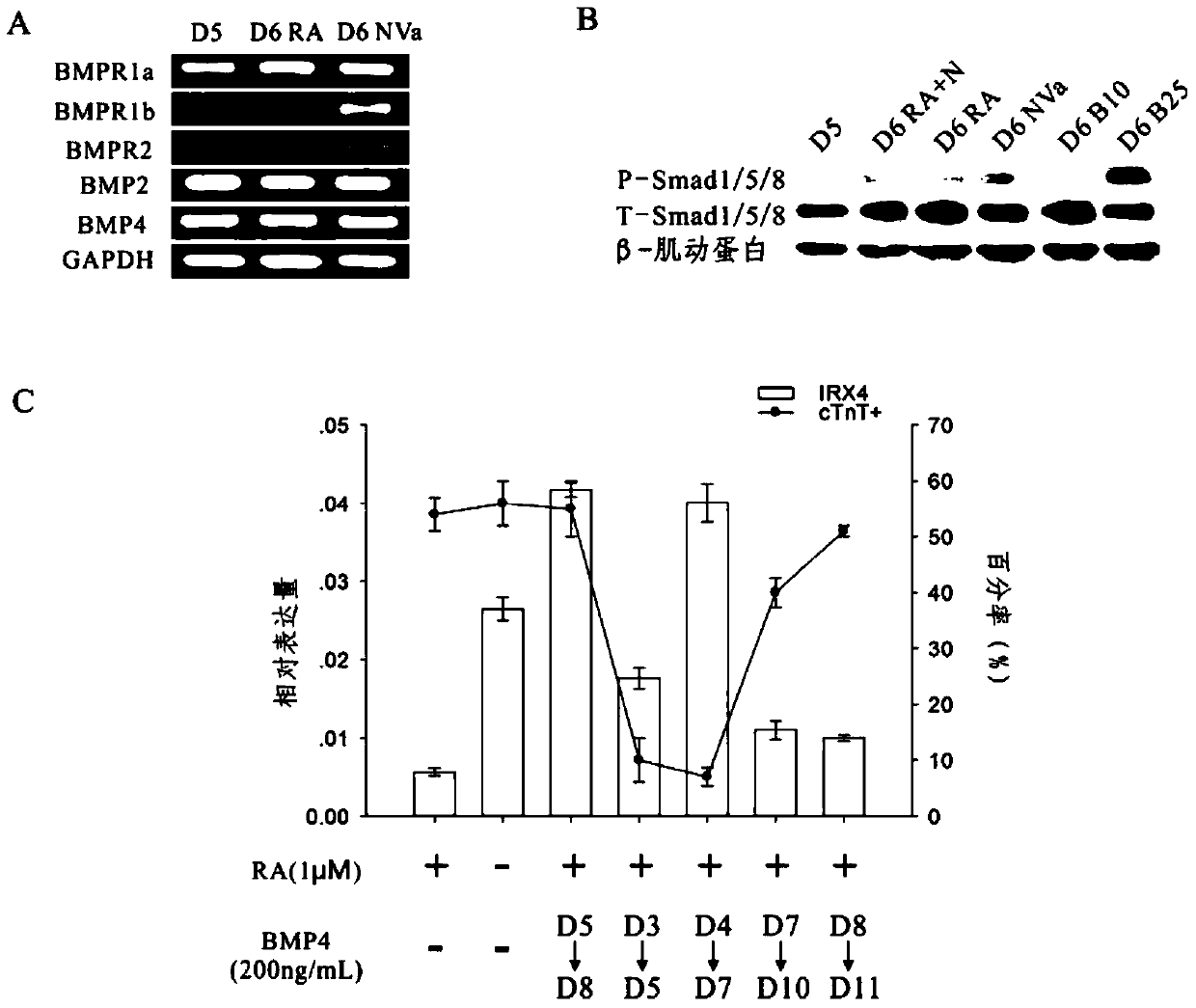
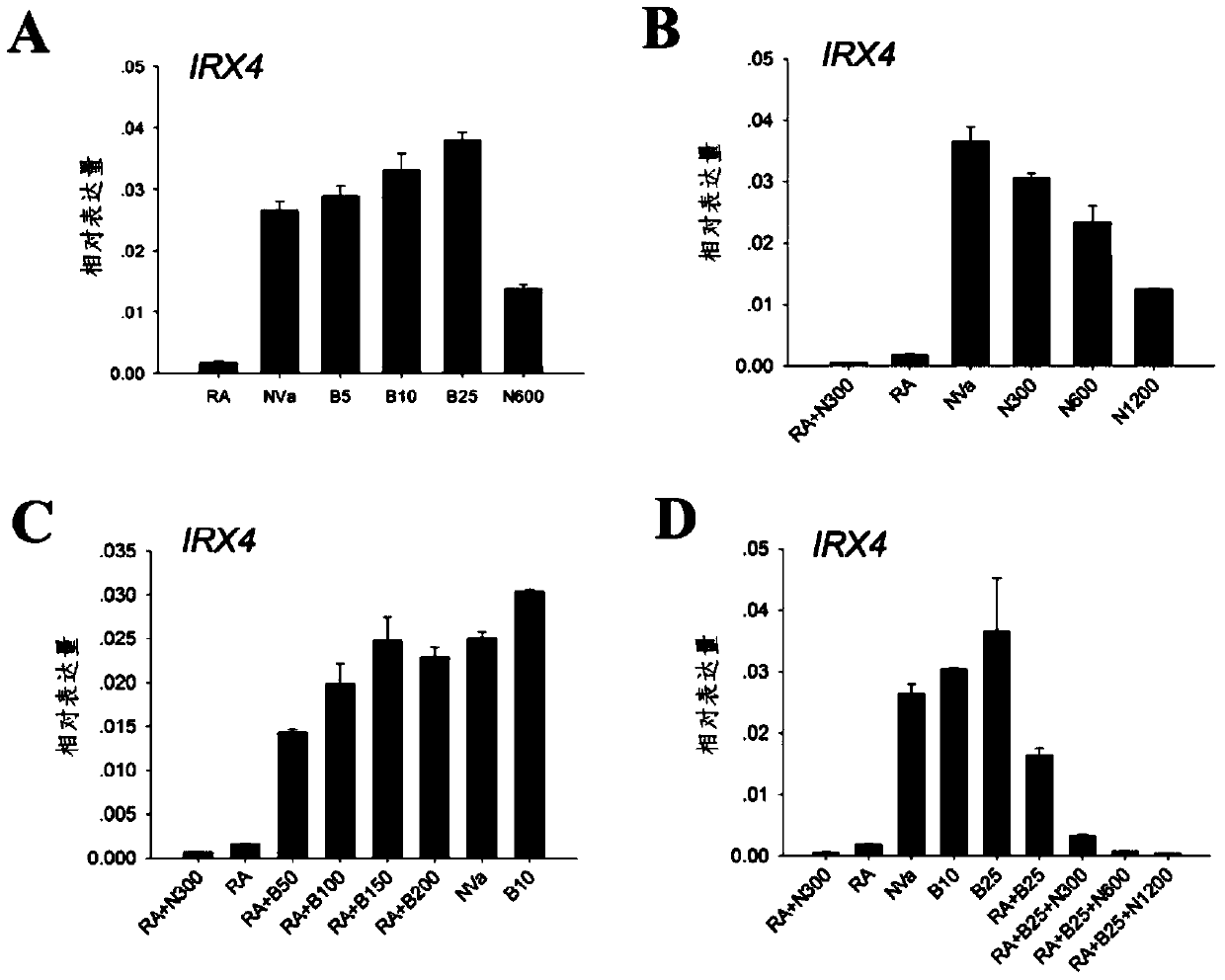
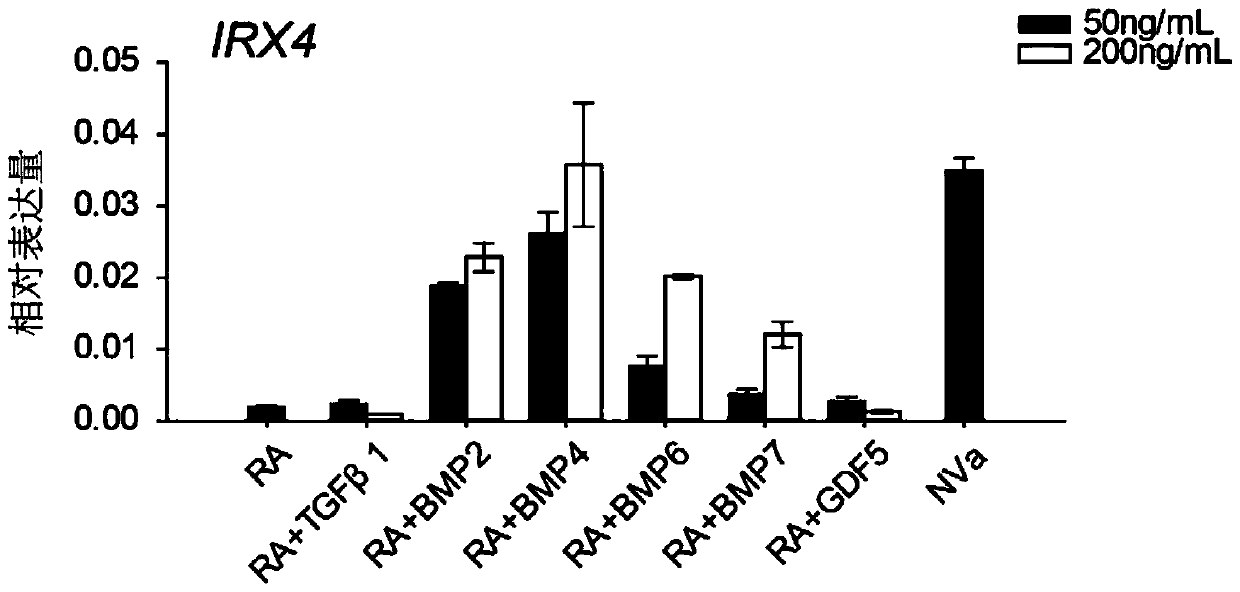
[0103] Example 1 The role of BMP / Smad1 / 5 / 8 signaling pathway in inducing myocardial precursor cells to differentiate into ventricular myocytes
[0104] 1 Studies have found that in the process of myocardial differentiation of stem cells, at the stage of determining the differentiation of cardiomyocyte subtypes, adding retinoic acid or its synthesis-required substrates such as vitamin A to the medium can induce stem cells to become atrial myocytes; If retinoic acid inhibitors are added to the medium at this time or vitamin A, the synthetic substrate of retinoic acid, is removed from the medium, stem cells can be directed to differentiate into ventricular myocytes 9 . By RT-PCR reaction, analyze the expression of BMP2 / 4 and its corresponding receptors in the differentiated human embryonic stem cells in the middle stage of differentiation, the results are as follows figure 1 As shown, both ligands and receptors of the BMP pathway are present in cultured cells. Western blot was ...
Embodiment 2
[0114]Example 2 Differentiation of Induced Pluripotent Stem Cells into Ventricular Myocytes in Vitro (Technical Scheme 1)
[0115] Spread human embryonic stem cells H7 on a culture dish containing gelatin (Gelatin), add RPMI1640 medium containing B27 (1×concentration) at 37°C CO 2 cultured in a cell culture incubator. The process of myocardial differentiation is Figure 9 As indicated, from day 0 to day 3, Activin A (10 ng / mL), BMP4 (6 ng / mL) and bFGF (6 ng / mL) were added to the medium. At the end of the third day, the medium was changed, and Noggin (300 ng / mL), an inhibitor of BMP2 / 4, was added at the same time. At the end of day 5, the medium was changed to RPMI1640 medium with B27 without vitamin A supplementation. At the same time, Wnt3a inhibitors DKK1 (300ng / mL) and BMP4 (10ng / mL) were added to the medium. At the end of day 8 of differentiation, the medium was changed to one containing only 300 ng / mL DKK1, and at the end of day 10 of differentiation without the addit...
Embodiment 3
[0116] Example 3 Differentiation of induced pluripotent stem cells into ventricular myocytes in vitro (technical scheme two)
[0117] Spread human embryonic stem cells H7 on a culture dish containing gelatin (Gelatin), add RPMI1640 medium containing B27 (1×concentration) at 37°C CO 2 cultured in a cell culture incubator. The process of myocardial differentiation is Figure 10 From day 0 to day 3, Activin A (10 ng / mL), BMP4 (6 ng / mL) and bFGF (6 ng / mL) were added to the medium as indicated. At the end of the 3rd day, the medium was changed, and Noggin (300 ng / mL), an inhibitor of BMP2 / 4, was added at the same time. At the end of day 5, the medium was changed to RPMI1640 medium with B27 without vitamin A supplementation. At the same time, the Wnt3a inhibitor DKK1 (300 ng / mL) and the retinoic acid receptor RARγ activator BMS961 (0.1 μM, purchased from Tocris) were added to the medium. At the end of day 8 of differentiation, change the medium to one containing only 300 ng / mL D...
PUM
 Login to View More
Login to View More Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com