Hydrobenzoate magnesium salt with anesthetic effect and preparation method of hydrobenzoate magnesium salt
A technology of hydroxybenzoate and anesthesia, which is applied in the field of medicine and chemical industry, can solve the problems of slow onset of action and harmful formaldehyde, and achieve the effects of quick recovery, safe clinical use, and good water solubility
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
Embodiment 1
[0025]
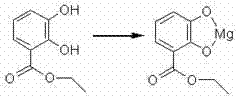
[0026] At room temperature, 2.4 g (0.0143 mol) of methyl 3,4-dihydroxybenzoate and 50 ml of distilled water were added to the reaction flask. 10 ml of an aqueous solution of 0.8 g (0.0143 mol) of magnesium hydroxide was added with stirring. After the addition was complete, the mixture was stirred for 2 hours, filtered, and the filtrate was fine-filtered again, and freeze-dried to obtain Compound 1 with a yield of 96.4%.
[0027] H nuclear magnetic resonance spectrum (D2O) of the compound: δ3.88 (CH3, s, 3H), 6.67 (CH, s, H), 7.27 (CH, s, H), 7.36 (CH, s, H) ppm. MS: m / z: 191.01 (M+1).
Embodiment 2
[0029]
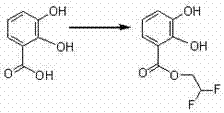
[0030] At room temperature, 2.6 g (0.0143 mol) of ethyl 3,4-dihydroxybenzoate and 50 ml of distilled water were added to the reaction flask. 10 ml of an aqueous solution of 0.8 g (0.0143 mol) of magnesium hydroxide was added with stirring. After the addition was complete, the mixture was stirred for 2 hours, filtered, and the filtrate was fine filtered again, and freeze-dried to obtain compound 2 with a yield of 97%.
[0031] The compound H NMR spectrum (D2O): δ1.3 (CH3, s, 3H), 4.29 (CH2, s, 2H), 6.67 (CH, s, H), 7.27 (CH, s, H), 7.36 ( CH, s, H) ppm. MS: m / z: 205.03 (M+1).
Embodiment 3
[0033]
[0034] At room temperature, 2.8 g (0.0143 mol) of 3,4-dihydroxypropyl benzoate and 50 ml of distilled water were added to the reaction flask. 10 ml of an aqueous solution of 0.8 g (0.0143 mol) of magnesium hydroxide was added with stirring. After the addition was completed, the mixture was stirred for 2 hours, filtered, and the filtrate was fine filtered again, and freeze-dried to obtain compound 3 with a yield of 95.3%.
[0035] The compound H nuclear magnetic resonance spectrum (D2O): δ0.96 (CH3, s, 3H), 1.79 (CH2, s, 2H), 4.25 (CH2, s, 2H), 6.67 (CH, s, H), 7.27 ( CH, s, H), 7.36 (CH, s, H) ppm. MS: m / z: 219.04 (M+1).
PUM
 Login to View More
Login to View More Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com