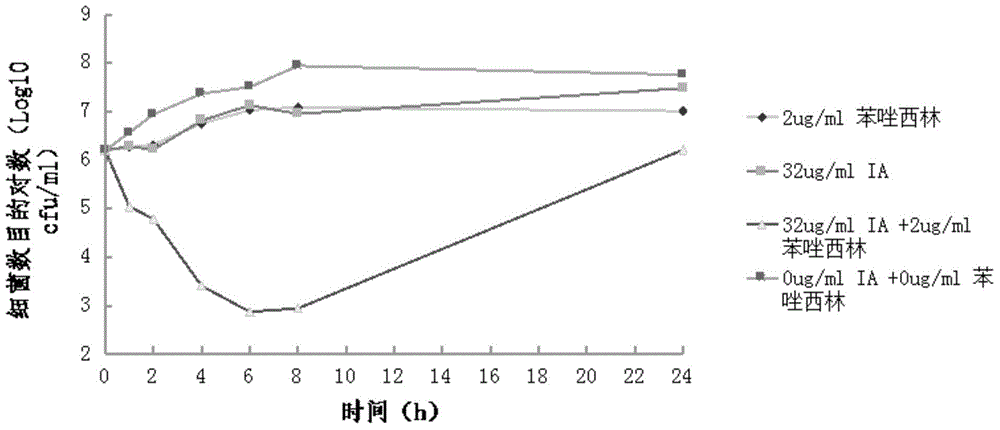
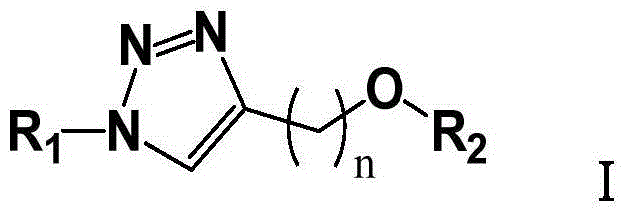
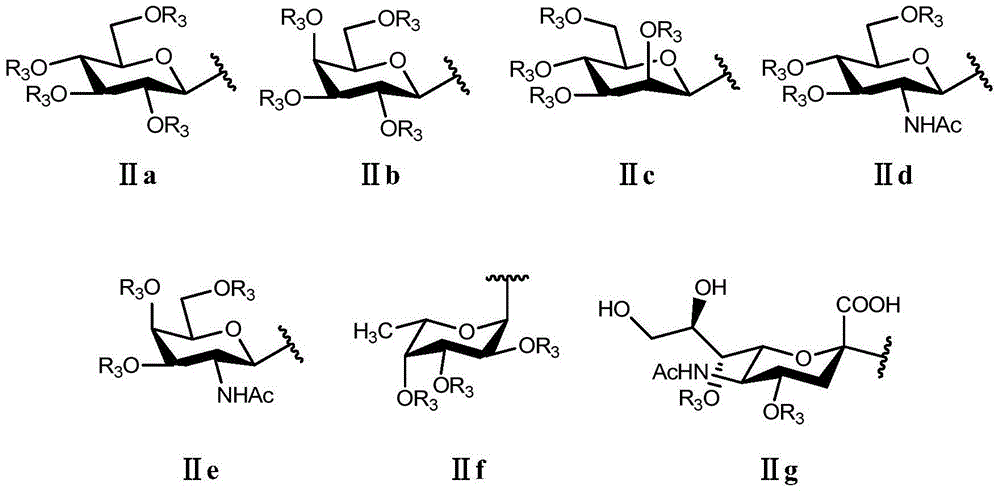
Triazole glycolipid derivatives and application thereof to synergically resist drug-resistant bacteria
A derivative, triazole technology, applied in the field of triazole glycolipid derivatives and their synergistic anti-drug-resistant bacteria, can solve problems such as adverse reactions of antibiotics
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
preparation example Construction
[0023] In the preparation method provided above, the azide compound (R 1 -N 3 ) can be prepared by classical end group bromination and azide substitution of various acetylated monosaccharides, see Alvarez S.G. & Alvarez, M.T. Synthesis, 1997, 413-414 for specific steps.
[0024] In the presence of NaH and at room temperature, the halogenated alkyne with the corresponding alcohol (R 2 OH) and a halogenated hydrocarbon (reaction medium) for at least 12 hours, followed by steps such as evaporation of the reaction medium, extraction, drying, filtration and concentration to obtain the intermediate
[0025] in anhydrous CuSO 4 In the presence of VcNa, inert gas (gas that does not participate in the reaction, such as nitrogen, etc.), and at room temperature, the azide compound (R 1 -N 3 ) and intermediates React for at least 12 hours, and the reaction product is washed successively, extracted, dried, filtered, concentrated and column chromatographed to obtain a product with...
Embodiment 1
[0028] (1) Synthesis of azidoglucose (compound shown in formula A) protected by all acetyl groups:
[0029]
[0030] Put glucose (3g, 0.017moL) into a 100mL flask, then slowly add Ac 2 O (11.9 mL) and HClO 4 (0.072mL) of the mixed solution, after the dropwise addition, remove the ice bath, react at room temperature, follow the reaction by TLC, until the raw material point disappears (at least about 6 hours), stop the reaction. Wash with ice saturated sodium bicarbonate and sodium chloride solution, extract with dichloromethane, and dry (MgSO 4 ), filtered and concentrated under vacuum to give the crude product (5.85 g, 90%). The obtained crude product was directly carried out to the next reaction without further purification.
[0031] Weigh all acetyl-protected glucose (0.53g, 0.001moL), add 30mL of dichloromethane as a solvent, then add 5mL of 30% HBr / AcOH solution to the system, react at room temperature, follow the reaction by TLC, until the starting material The spo...
Embodiment 2
[0042] Except replacing the n-dodecyl alcohol in the step (2) of Example 1 with n-tetradecyl alcohol, other raw materials used, reagents and steps are similar to those in Example 1, and the compound shown in formula IB can be obtained:
[0043]
[0044] 1 H NMR (400MHz, DMSO) δ8.32(s, 1H), 5.57(d, J=9.3Hz, 1H), 5.44(d, J=5.9Hz, 1H), 5.35(s, 1H), 5.23(d ,J=5.3Hz,1H),4.70(s,1H),4.54(s,2H),3.86–3.74(m,2H),3.50(d,J=6.4Hz,4H),3.48(s,1H) ,3.33–3.25(m,1H),1.30(s,24H),0.91(t,J=6.7Hz,3H).
[0045] 13 C NMR (101MHz, DMSO) δ143.9, 123.0, 87.4, 79.8, 79.2, 78.9, 78.6, 76.9, 71.9, 69.7, 69.5, 63.2, 60.7, 54.8, 48.5, 31.2, 29.1, 29.0, 28.9, 28.7, 25.6, 22.0 ,13.9.
PUM
 Login to View More
Login to View More Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com