Polylactone preparation method
A technology of polylactone and cyclic lactone, applied in the field of polylactone, can solve the problems of complicated synthesis of bifunctional catalysts, and achieve the effects of wide application type, simple synthesis and narrow molecular weight distribution
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
Embodiment 1
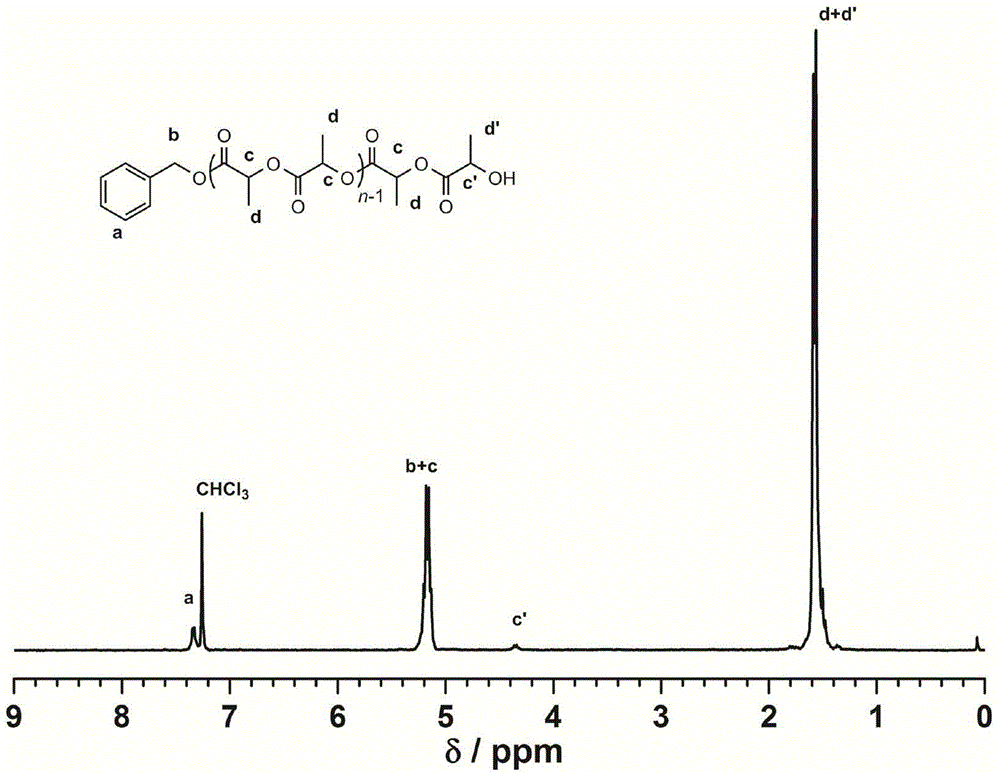
[0039] Square amide (Sq1) (26.8mg, 0.05mmol, 1.0equiv), (-)-spartine (11.7mg, 0.05mmol, 1.0equiv), benzyl alcohol (5.2μL, 0.05mmol, 1.0equiv) and L - Lactide (216mg, 1.5mmol, 30equiv) was added to the reaction flask, dissolved with 1.5mL of dichloromethane, and stirred at room temperature for 2 hours under the protection of Ar. Add benzoic acid to terminate the reaction, concentrate the reactant and pour it into methanol, filter the precipitate and dry to constant weight to obtain 148 mg of white solid with a transformation rate of 98.2%. The number average molecular weight of polylactic acid is M n 4330g mol -1 , the dispersion PDI is 1.05, 1 H NMR (300MHz, CDCl 3 ):δ(ppm)1.57(m,3H×n,(–CH 3 ) n ),4.34(m,–CH(CH 3 )OH), 5.13-5.21(q, 1H×n-1, J=7.0, –CH(CH 3 )O–; 2H, ArCH 2 O–),7.33-7.34(m,5H,aromatic).
Embodiment 2
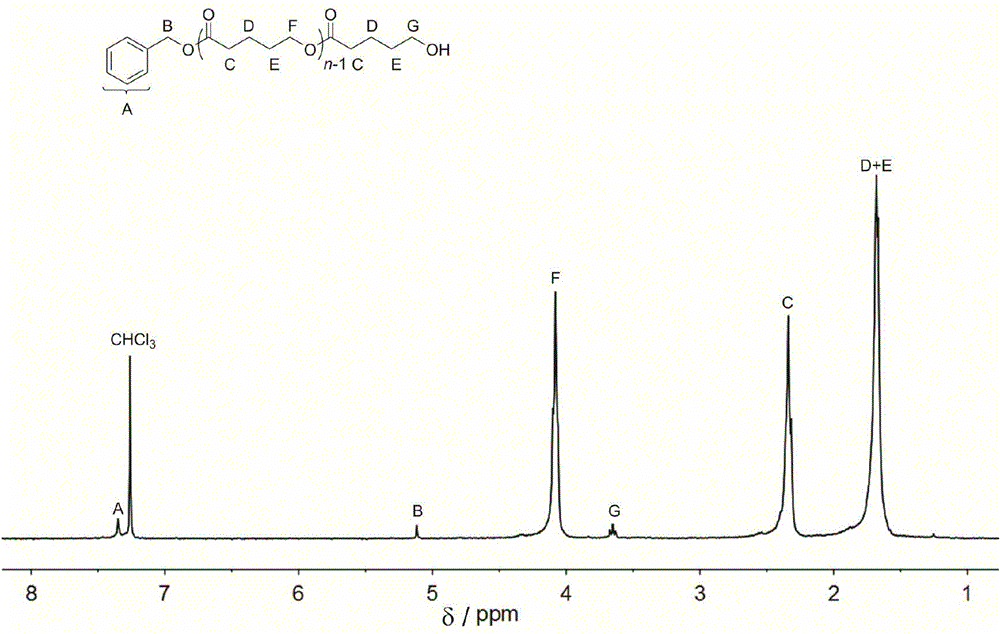
[0041]Square amide (Sq1) (26.8mg, 0.05mmol, 1.0equiv), (-)-spartenine (11.7mg, 0.05mmol, 1.0equiv), benzyl alcohol (5.2μL, 0.05mmol, 1.0equiv) and δ - Add valerolactone (150mg, 1.5mmol, 30equiv) into the reaction flask, dissolve it with 1.5mL of dichloromethane, and react with stirring at room temperature for 2 hours under the protection of Ar. Add benzoic acid to terminate the reaction, concentrate the reactant and pour it into methanol, filter the precipitate and dry to constant weight to obtain 102 mg of white solid with a transformation rate of 93.5%. The number average molecular weight of polylactic acid M n 4320g mol -1 , the dispersion PDI is 1.07; 1 H NMR (300MHz, CDCl3): δ (ppm) 1.68 (m, 2H×n, (–CH 2 CH 2 CH 2 O–) n ),1.70(m,2H×n,(–COCH 2 –CH 2 CH 2 –) n ),2.34(t,2H×n,J=6.8Hz,(–OCOCH 2 CH 2 –) n ),3.65(t,2H,J=6.1Hz,–CH 2 CH 2 OH), 4.08(t, 2H×n, J=5.5Hz, (–CH 2 CH 2 O-) n ),5.12(s,2H,ArCH 2 O), 7.32–7.39 (m, 5H, aromatic).
Embodiment 3
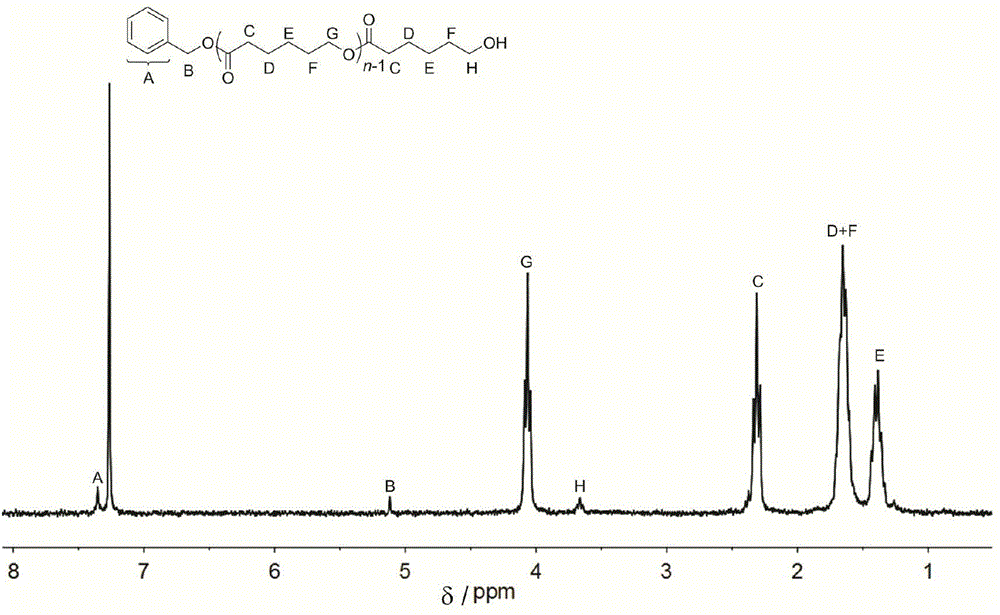
[0043] Square amide (Sq1) (26.8mg, 0.05mmol, 1.0equiv), (-)-spartenine (11.7mg, 0.05mmol, 1.0equiv), benzyl alcohol (5.2μL, 0.05mmol, 1.0equiv) and ε - Caprolactone (171 mg, 1.5 mmol, 30 equiv) was added to the reaction flask, dissolved with 1.5 mL of dichloromethane, and stirred at room temperature for 6 hours under the protection of Ar. Add benzoic acid to terminate the reaction, concentrate the reactant and pour it into methanol, filter the precipitate and dry to constant weight to obtain 94.5 mg of white solid with a transformation rate of 92.3%. The number average molecular weight of polylactic acid is M n 3530g mol -1 ;, the dispersion PDI is 1.10; 1 H NMR (CDCl 3 ),δ(ppm),1.38(m,2H×n,(–CH 2 CH 2 CH 2 CH 2 CH 2 –) n ),1.65(m,2H×n,(–CH 2 CH 2 CH 2 O–) n ),1.68(m,2H×n,(–COCH 2 CH 2 –CH 2 –) n ),2.31(t,2H×n,J=7.3Hz,(–OCOCH 2 CH 2 –) n ),3.66(t,2H,J=6.6Hz,–CH 2 CH 2 OH), 4.06(t, 2H×n, J=6.9Hz, (–CH 2 CH 2 O–) n ),5.12(s,2H,ArCH 2 O),7.32–7.40(m,5H,ar...
PUM
| Property | Measurement | Unit |
|---|---|---|
| Number average molecular weight | aaaaa | aaaaa |
| Number average molecular weight | aaaaa | aaaaa |
| Number average molecular weight | aaaaa | aaaaa |
Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com