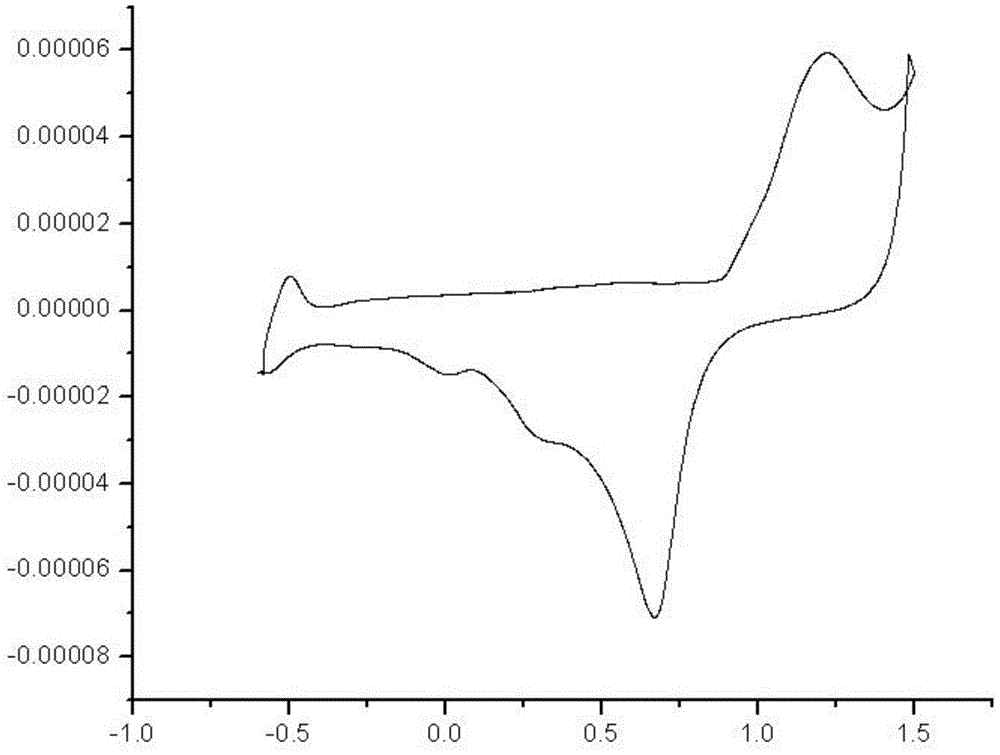
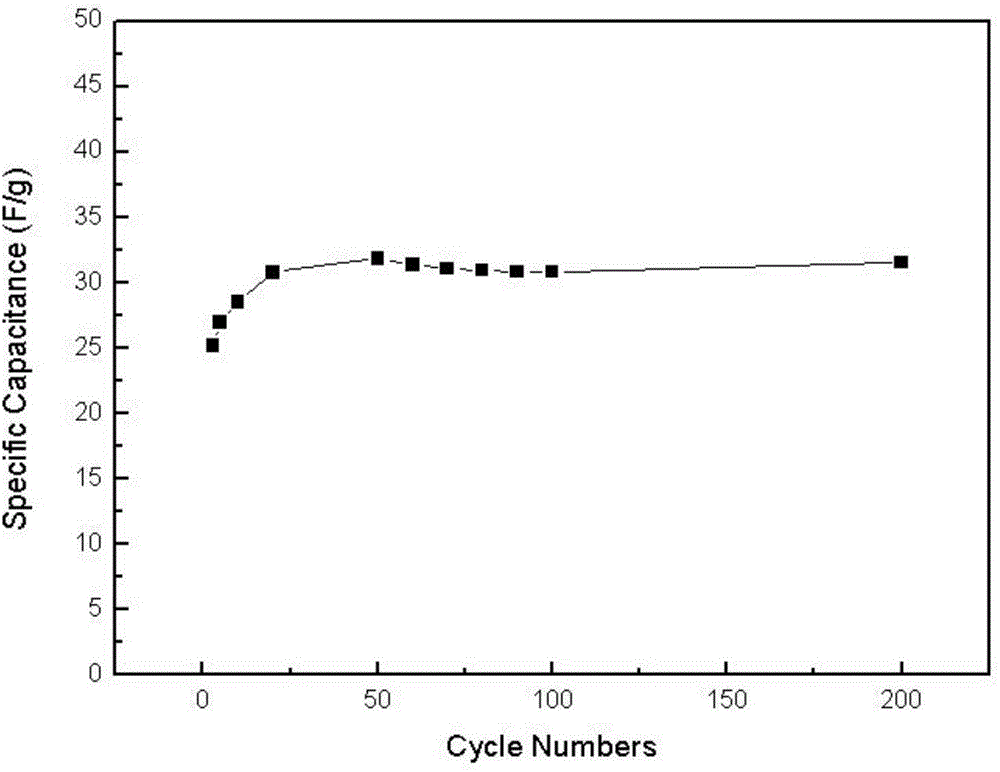
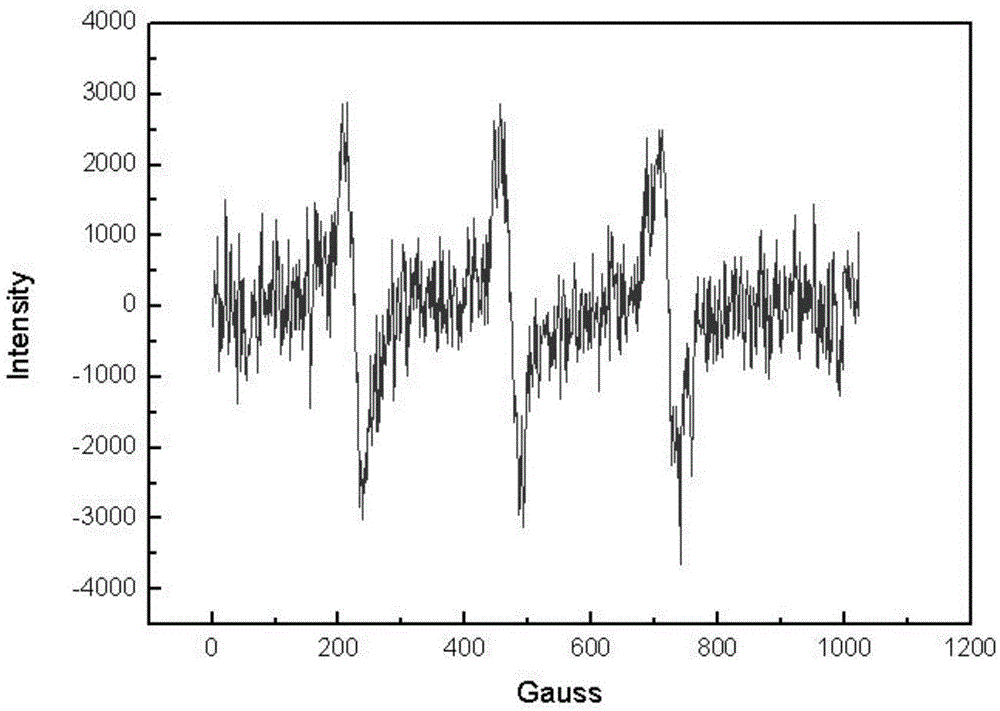
Isoindole nitrogen oxide free radical modified polypyrrole and preparation method and application thereof
An indene nitric oxide and free radical technology is applied in the field of isoaza indene nitric oxide radical modified polypyrrole and its preparation, which can solve the problem that energy density and cycle stability are difficult to meet application requirements, poor solubility and processing performance, and poor filling performance. Discharge specific capacity is low and other problems, to achieve the effect of good dissolution and processing performance, high power density, long cycle life
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
Embodiment 1
[0043] poly(3,4-diisoazindene nitroxide radical amine-pyrrole)
[0044] Under ice bath conditions, dissolve a certain amount of FeCl in 100mL deionized water 3 .6H 2 O and dopant, fully stirred to dissolve the solid, (tert-butoxycarbonyl)-aminotriisopropylchlorosilylpyrrole (0.5g, 1.5mmol) was added to the above solution, continued to stir, and passed N 2 Protect. React at room temperature for 6 hours, filter, remove the solvent and water under reduced pressure, and dry in vacuo to obtain poly(tert-butoxycarbonyl)-amino-triisopropylchlorosilylpyrrole.
[0045] Add 1.0 mL of trifluoroacetic acid to poly(tert-butoxycarbonyl)-amino-triisopropylchlorosilylpyrrole (0.168 g, 0.5 mmol) in dichloromethane (1.5 mL) and anisole (1.0 mL) After the reaction, the reaction solution was poured into 10 mL of saturated sodium bicarbonate solution and stirred at room temperature for 1 h, and the solvent and water were removed under reduced pressure. Vacuum drying gave polyamino-triisopropyl...
Embodiment 2
[0050] Poly(3-isozaindene nitroxide radical vinyl-pyrrole)
[0051] (2,5-dibromopyrrole-3-methylene)-phosphate (0.117g, 0.3mmol) was dissolved in DMF (5mL), and sodium methoxide (0.016g in 2mL DMF) was added to the reaction under ice cooling in the liquid. 5-formaldehyde-1,1,3,3-tetramethylisozaindene nitrogen oxide radical (0.059 g, 0.27 mmol) was added to the reaction solution for 30 minutes of reaction. After the reaction, the solution was poured into ice water, extracted with ether, washed with water, dried over anhydrous magnesium sulfate, filtered, the solvent was removed in vacuo, purified by column chromatography, the solvent was removed under reduced pressure, and dried in vacuo to obtain the free radical modified pyrrole.
[0052] Free radical modified pyrrole (0.053g, 0.12mmol) was dissolved in 5mL tetrahydrofuran, dried under nitrogen, CH 3 MgBr (0.06mL, 0.12mmol, 2.1mol / L ether solution) was added dropwise to the reaction flask, and Ni(dppp)Cl 2 (0.07mg, 1%) wa...
Embodiment 3
[0054] (3-Isozaindene nitroxide radical vinyl-pyrrole)-pyrrole copolymer
[0055] (2,5-dibromopyrrole-3-methylene)-phosphate (0.117g, 0.3mmol) was dissolved in DMF (5mL), and sodium methoxide (0.016g in 2mL DMF) was added to the reaction under ice cooling in the liquid. 5-formaldehyde-1,1,3,3-tetramethylisozaindene nitrogen oxide radical (0.059 g, 0.27 mmol) was added to the reaction solution for 30 minutes of reaction. After the reaction, pour the solution into ice water, extract with ether, wash with water, dry over anhydrous magnesium sulfate, filter, remove the solvent under reduced pressure, purify by column chromatography (dichloromethane), remove the solvent under reduced pressure, and dry in vacuo to obtain free radical modification pyrrole.
[0056] Free radical modified pyrrole (0.053g, 0.12mmol) and 2,5-(3-butyltin)pyrrole (0.077g, 0.12mmol) were dissolved in 3mL THF, Pd 2 (dba) 3 (0.0025g, 0.0028mmol), CuI (0.002g, 0.013mmol) and AsPh 3 (0.0016g, 0.005mmol) wa...
PUM
 Login to View More
Login to View More Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com