Acid addition salt of afatinib and its crystal form, its preparation method and pharmaceutical composition
A technology of afatinib and tinib ethanedisulfonate, applied in the directions of drug combination, carboxylate preparation, sulfonate preparation, etc., can solve the problem of affecting the uniformity of preparations, reducing the content of pharmaceutical active substances, and limiting hygroscopicity and other problems, to achieve the effect of reducing the risk of curative effect decline and safety risk, improving the uniformity of the preparation, and having a good crystal morphology.
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
preparation example 1
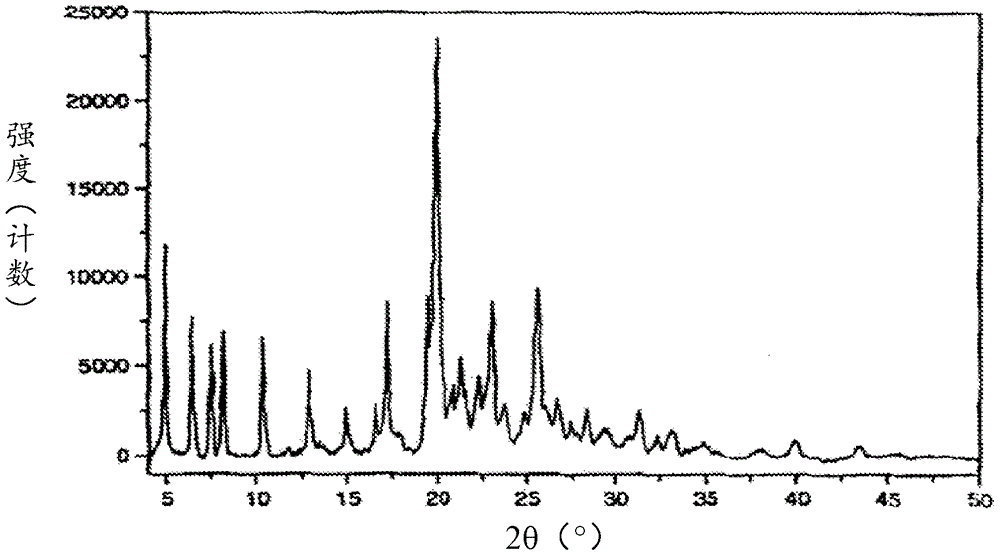
[0321] Preparation Example 1 Preparation of the prior art crystal form of afatinib dimaleate
[0322] Take 100mg of afatinib free base and add 1.5mL of ethanol to stir and dissolve, heat to 70°C, take 50mg of maleic acid and add 0.4mL of ethanol to stir and dissolve, slowly add the ethanol solution of maleic acid to the solution of afatinib free base In the ethanol solution, stirring, after the solid precipitated, the reaction solution was cooled to room temperature, stirred at room temperature for 2-3h, filtered, and vacuum-dried at 40°C overnight to obtain afatinib dimaleate.
[0323] XRPD analysis such as figure 2 As shown, it is consistent with the prior art crystal form of afatinib dimaleate disclosed in CN1867564B.
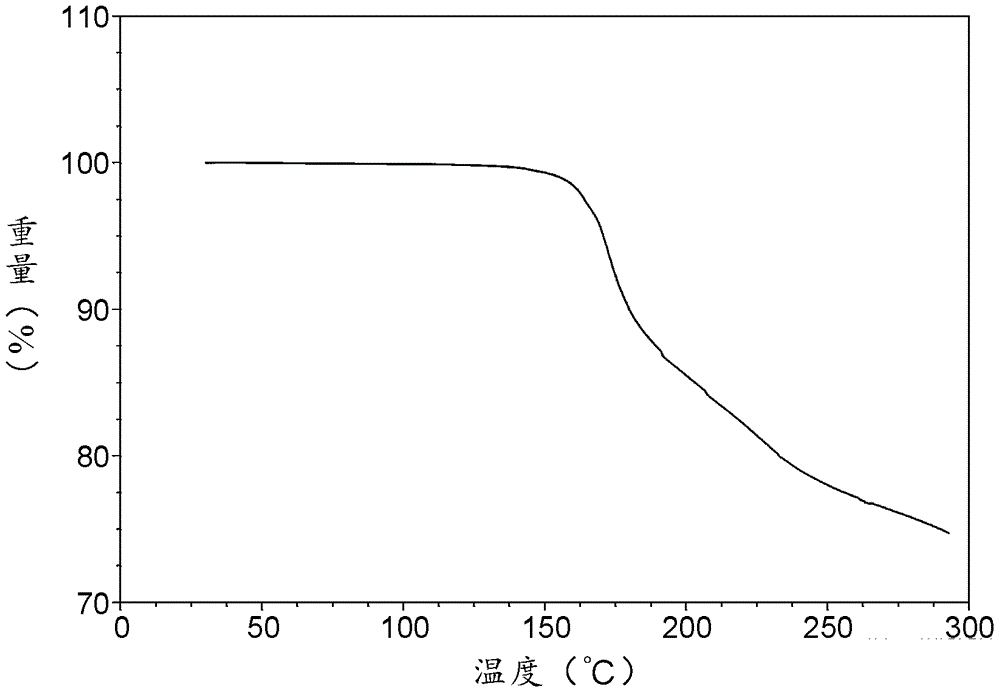
[0324] TGA spectrum such as image 3 As shown, it shows that the decomposition temperature of the salt is 164.1°C.
[0325] DSC spectrum such as Figure 4 , showing that the salt has a melting point of 166.2°C.
[0326] DVS isotherm adsorption curve...
Embodiment 1
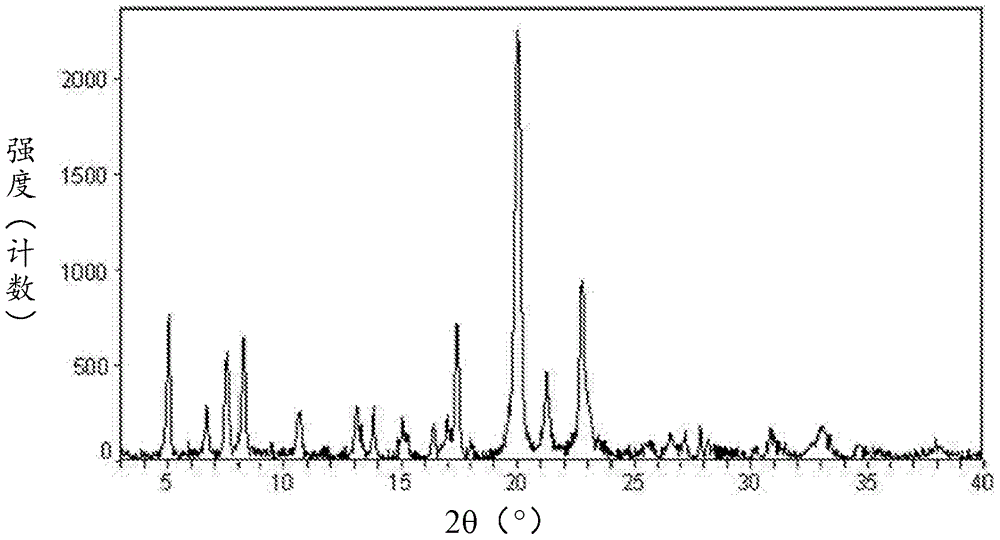
[0327] Example 1 Preparation of afatinib edisylate
[0328] Take 50mg of afatinib free base and add 1mL of ethyl acetate and stir to dissolve, take 19.6mg of ethanedisulfonic acid and add 2mL of ethyl acetate and stir to dissolve, slowly add the ethyl acetate solution of ethanedisulfonic acid to the free base of afatinib A slurry was formed in the ethyl acetate solution of the base, stirred, reacted overnight at room temperature, and a solid precipitated out, and was spin-dried under vacuum at 40°C to obtain 68 mg of afatinib edisylate, with a yield of 97.7%.
Embodiment 2
[0329] Example 2 Preparation of E1 crystal form afatinib edisylate
[0330] Add 50 mg of afatinib free base to 1 mL of acetonitrile and stir to dissolve, take 19.6 mg of ethanedisulfonic acid to add 2 mL of acetonitrile and stir to dissolve, slowly add the acetonitrile solution of ethanedisulfonic acid to the acetonitrile solution of afatinib free base to form The slurry was stirred, reacted overnight at room temperature, and solids precipitated out, filtered, and dried under vacuum at 40°C overnight to obtain 62 mg of afatinib edisylate in E1 crystal form, with a yield of 89%.
PUM
| Property | Measurement | Unit |
|---|---|---|
| Decomposition temperature | aaaaa | aaaaa |
| Melting point | aaaaa | aaaaa |
| Decomposition temperature | aaaaa | aaaaa |
Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com