Manufacturing method of nitrile and corresponding amine thereof
A manufacturing method and amide technology, which are applied in chemical instruments and methods, dehydration preparation of carboxylic acid amides, preparation of organic compounds, etc., can solve the problems of inconsistent production concepts, loss of reaction materials, side reactions, and high production costs.
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
Embodiment
[0137] The following examples will be used to illustrate the present invention in further detail, but the present invention is not limited to these examples.
[0138] Amide Intermediate Preparation Example A
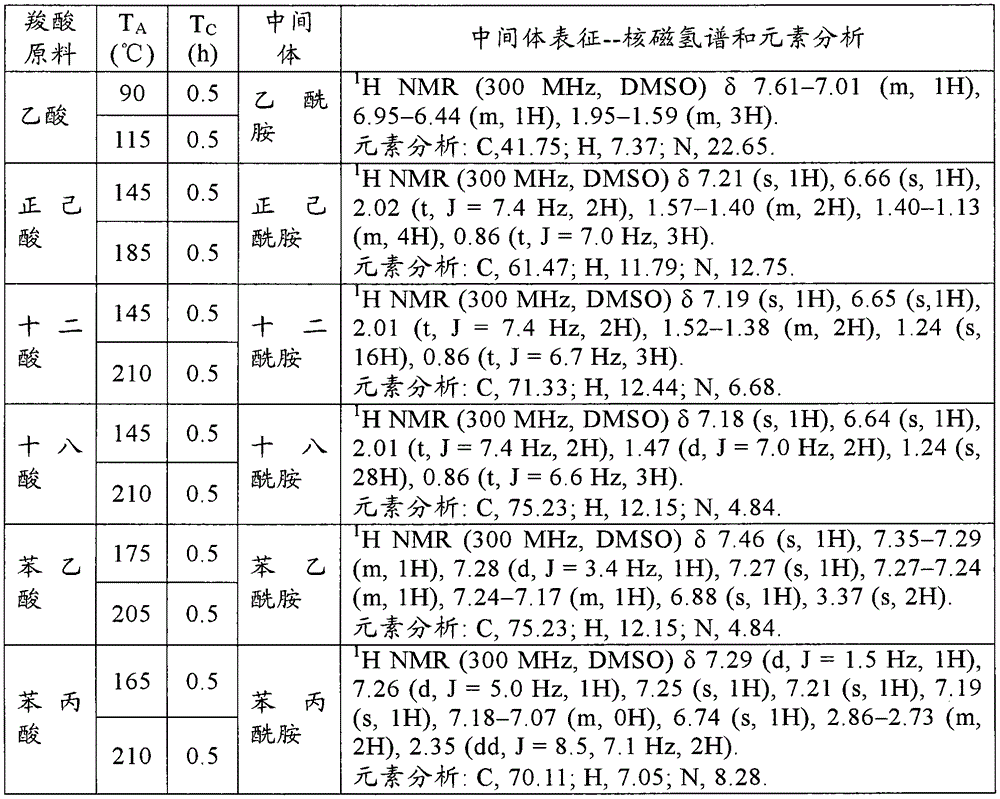
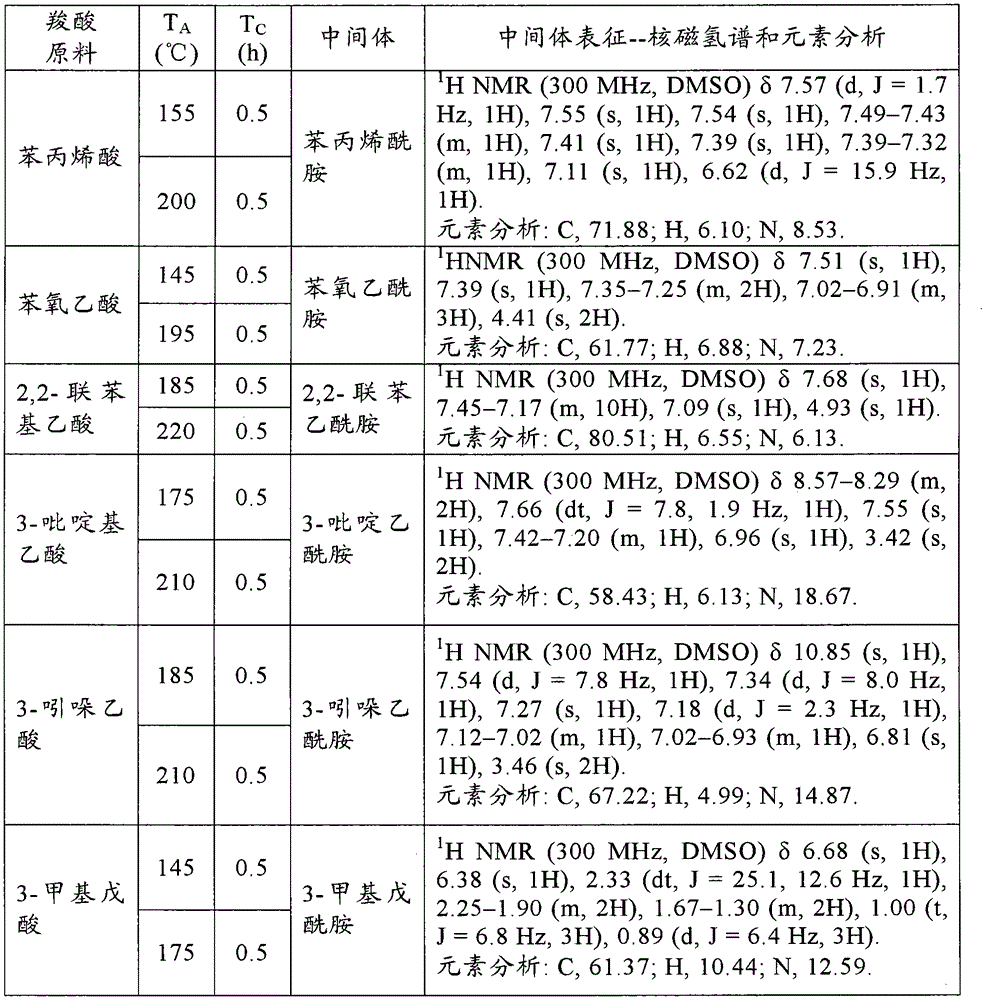
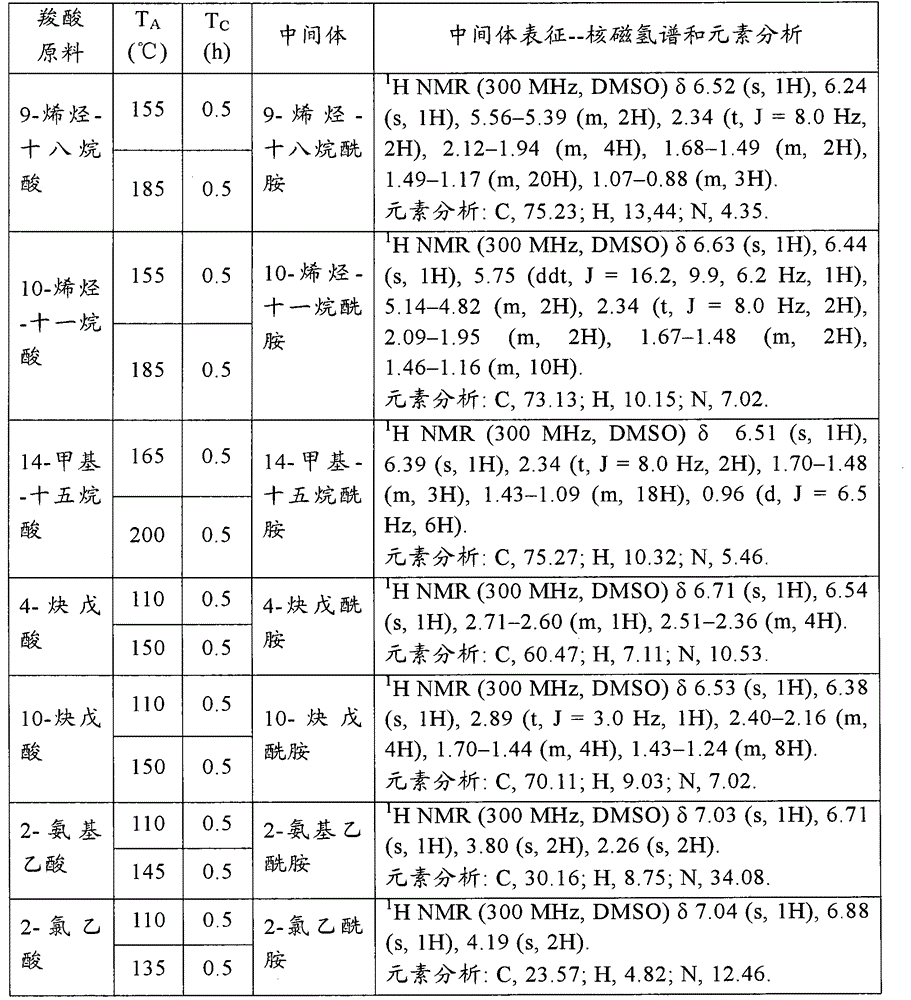
[0139] Add 500g carboxylic acid raw material (chemically pure) in 1L open reactor, open and stir (600r / min), constantly pass into ammonia gas (chemically pure, water content is 5.1wt% in the carboxylic acid raw material from the reactor bottom, flow rate is 100g / min). Make the reaction at the reaction temperature T A T C After 1 hour, the ammonia gas was stopped. The contents of the reactor were sampled for 1H NMR and elemental analysis to characterize the amide intermediate. The specific reaction conditions and characterization results are shown in the following Table A-1, Table A-2, Table A-3, Table A-4, Table A-5 and Table A-6. These characterization results show that the obtained amide intermediate has extremely high purity (over 99%).
[0140] In this embodime...
preparation Embodiment A
[0154] Example A was prepared following the amide intermediate. Close described reaction kettle (when the boiling point of amide intermediate at normal pressure is equal to or lower than following reaction temperature T B ) or keep the reactor open (when the boiling point of the amide intermediate at normal pressure is higher than the following reaction temperature T B time), continue to stir (600r / min), change the reaction temperature to T B , at the reaction temperature T B keep T down D After 1 hour, the reaction was essentially complete. Then, close the reactor and connect the vacuum pump to make the vacuum in the reactor reach 20-50mbar (adjust accordingly according to the difference of the nitrile product type), and use the distillate as the nitrile product. Calculate the yield of the nitrile product, and take a sample for proton nuclear magnetic spectrum and elemental analysis to characterize the obtained nitrile product. The specific reaction conditions and charac...
preparation Embodiment A1
[0169]Example A was prepared following the amide intermediate. Seal described reactor, open stirring (600r / min), temperature of reaction is changed to T B . The reactor was connected to a vacuum pump, and the vacuum in the reactor was gradually reduced from 500 mbar until a trace amount (up to about 0.5 wt %) of the amide intermediate was detected in the effluent. Maintain the vacuum degree, the reaction time T D After 1 hour, the reaction was essentially complete. The distillate was taken as the nitrile product. Calculate the yield of the nitrile product, and take a sample for proton nuclear magnetic spectrum and elemental analysis to characterize the obtained nitrile product. The specific reaction conditions and characterization results are shown in the following tables A1-7, A1-8, A1-9, A1-10, A1-11 and A1-12. These characterization results show that the obtained nitrile product has a high purity (above 92%).
[0170] In these nitrile product preparation examples, opt...
PUM
 Login to View More
Login to View More Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com