Method for preparing 2,5-dimethyl furan by catalyzing selective hydrodeoxygenation of 5-hydroxymethyl furfural
A technology of hydroxymethylfurfural and dimethylfuran, which is applied in the field of biofuel preparation, can solve the problems of disadvantages, long reaction time, and low yield, and achieve the effects of less waste discharge, simple operation process, and high yield
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
Embodiment 1
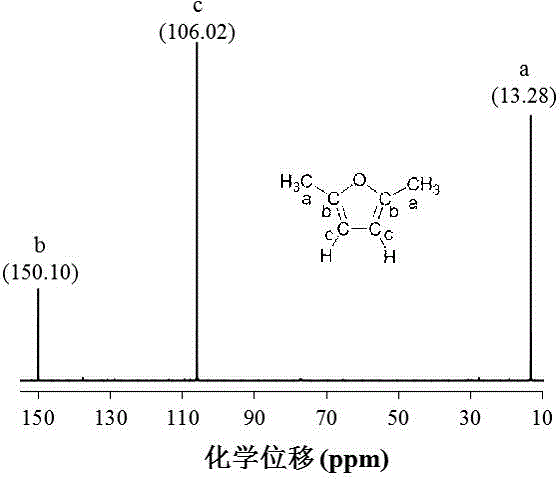
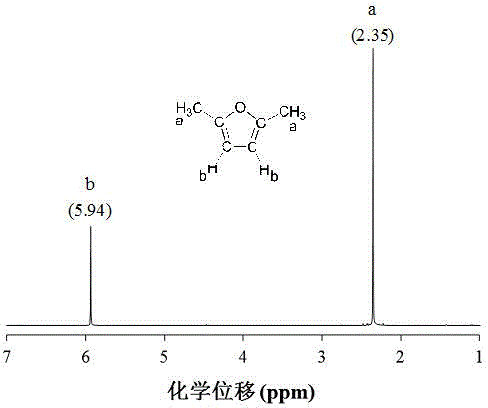
[0023] Example 1: Add 2.5% 5-HMF, 30% Ru / AC and 20 g THF into the autoclave, seal it with H 2 Replace the air in the kettle; then H 2 The pressure was adjusted to 20 bar, the temperature was raised to 180 °C, and the reaction was carried out at a stirring speed of 500 rpm for 120 min; after the reaction was completed, the high-pressure reactor was rapidly lowered to room temperature and opened, and the hydrodeoxygenation reaction liquid was filtered and detected by a gas chromatograph It can be seen that the conversion rate of 5-HMF is 90.7%, and the yield of 2,5-DMF is 80.6%; then the filtrate is subjected to atmospheric distillation at 66 °C to obtain THF and the initial steamed residual liquid, and the initial steamed residual liquid is then Carried out vacuum distillation at 75 °C to obtain 2,5-DMF and H 2 A mixture of O, 2,5-DMF and H 2 After O is automatically stratified, the upper layer is purified 2,5-DMF, which can be seen by nuclear magnetic resonance spectromete...
Embodiment 2
[0024] Example 2: Add 3.75% 5-HMF, 20% Ru / AC and 25 g THF into the autoclave, seal it with H 2 Replace the air in the kettle; then H 2 The pressure was adjusted to 25 bar, the temperature was raised to 220 °C, and the reaction was carried out at a stirring speed of 600 rpm for 100 min; after the reaction was completed, the high-pressure reactor was quickly lowered to room temperature and opened, and the hydrodeoxygenation reaction solution was filtered and detected by a gas chromatograph It can be seen that the conversion rate of 5-HMF is 100%, and the yield of 2,5-DMF is 94.5%; then the filtrate is subjected to atmospheric distillation at 66 °C to obtain THF and the initial steamed residual liquid, and the initial steamed residual liquid is then Carried out vacuum distillation at 75 °C to obtain 2,5-DMF and H 2 A mixture of O, 2,5-DMF and H 2 After automatic stratification of O, the upper layer is purified 2,5-DMF, and the purity of 2,5-DMF is 98% as detected by NMR spect...
Embodiment 3
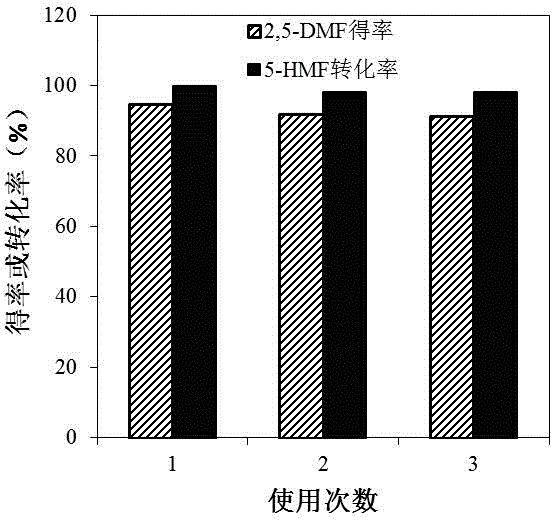
[0025] Example 3: Add 2.5% 5-HMF, 40% Ru / AC and 20 g THF into the autoclave, seal it with H 2 Replace the air in the kettle; then H 2 Adjust the pressure to 20 bar, heat up to 200 °C, and react at a stirring speed of 400 rpm for 120 min; after the reaction, the high-pressure reactor was quickly lowered to room temperature and opened, and the hydrodeoxygenation reaction liquid was filtered and detected by gas chromatography It can be seen that the conversion rate of 5-HMF was 100%, and the yield of 2,5-DMF was 94.7%; the filtered Ru / AC was washed 10 times with 5 mL THF, and dried in vacuum at 65 °C for 24 h; The dried Ru / AC was added to fresh THF and subjected to the next round of hydrodeoxygenation reaction under the same reaction conditions as above, from image 3 It can be seen that the conversion rate of 5-HMF can still reach more than 98% when Ru / AC is used for the second and third times, and the yield of 2,5-DMF can also reach more than 91%; After the reaction solutio...
PUM
| Property | Measurement | Unit |
|---|---|---|
| boiling point | aaaaa | aaaaa |
Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com