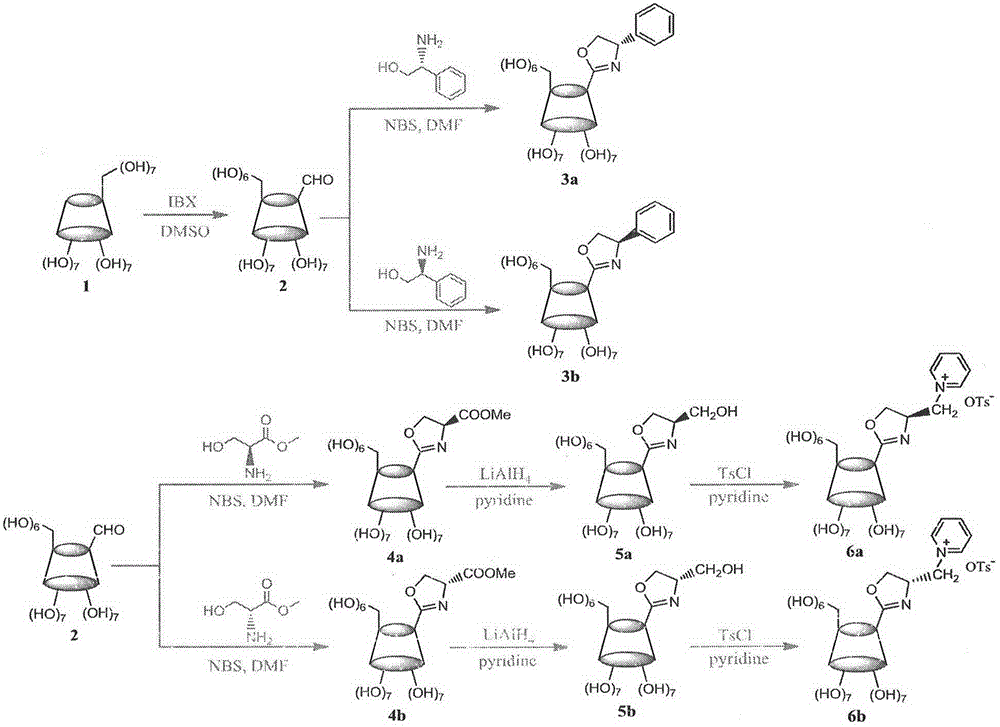
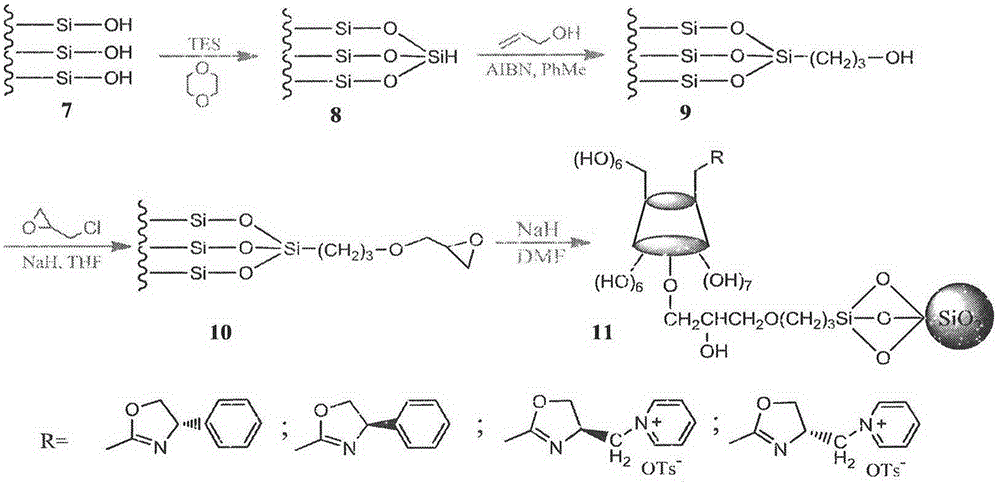
Cyclodextrin derivative containing oxazoline segments as well as preparation and application of hydrogenated silica gel stationary phase bonded with cyclodextrin derivative
A technology containing oxazoline groups and cyclodextrins, applied in other chemical processes, organic chemistry, organic chemistry methods, etc., can solve problems such as shedding and interference in the separation process of reversed-phase chromatography, and achieves low polarity and is not easy to drag. Tail, high separation effect
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
Embodiment example 1
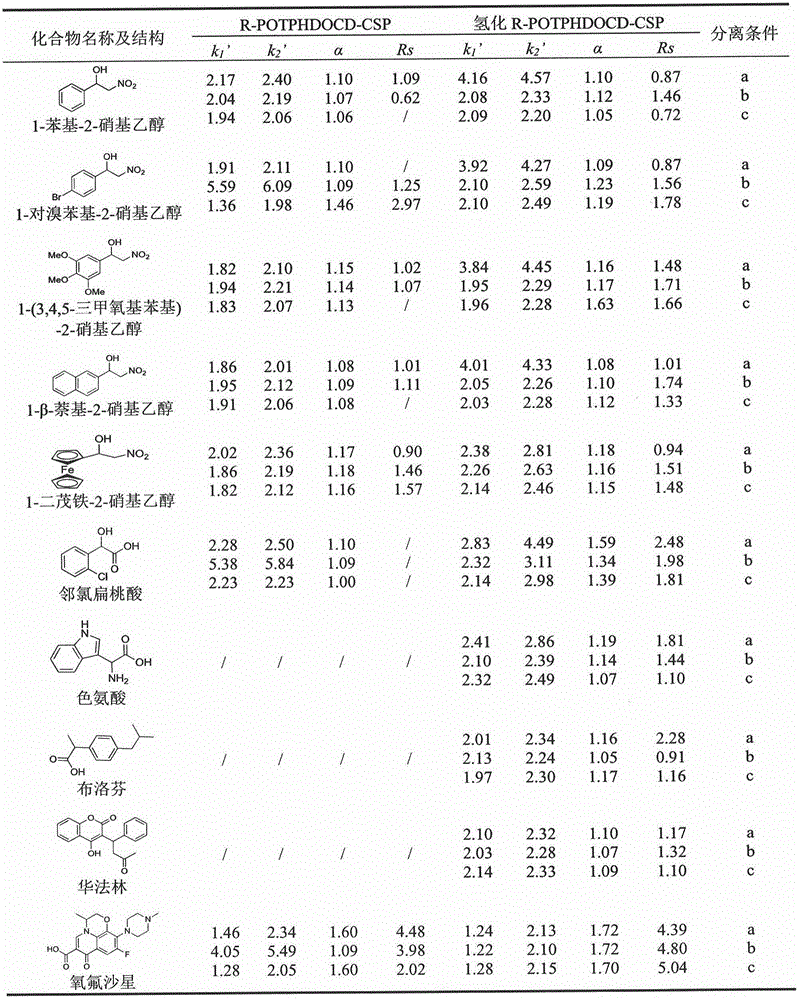
[0024] The suspensions of R-POTPHDOCD-CSP and hydrogenated-R-POTPHDOCD-CSP were pumped into two chromatographic columns of 250mm×4.6mm (i.d.) specifications by homogenization method respectively, and were mixed in polar organic mode (acetonitrile / methanol / acetic acid / triethylamine) to measure the chromatographic properties of the two columns; the mobile phase was filtered through a 0.45 μm organic microporous membrane before use, and vacuum degassed for 30 minutes; the sample was dissolved in chromatographically pure methanol and filtered through a 0.45 μm needle filter ; The mobile phase flow rate is 0.6mL / min; The detection slope length is 254nm. See attached for separation data image 3 .
Embodiment example 2
[0026] The suspensions of R-POTPHDOCD-CSP and hydrogenated-R-POTPHDOCD-CSP were pumped into two chromatographic columns of 250mm×4.6mm (i.d.) specifications by homogenization method respectively, and were mixed in polar organic mode (acetonitrile / methanol / acetic acid / triethylamine) to measure the chromatographic properties of the two columns; the mobile phase was filtered through a 0.45 μm organic microporous membrane before use, and vacuum degassed for 30 minutes; the sample was dissolved in chromatographically pure methanol and filtered through a 0.45 μm needle filter ; The mobile phase flow rate is 0.6mL / min; The detection slope length is 254nm. See attached for separation data Figure 4 .
Embodiment example 3
[0028] The suspensions of R-POTPHDOCD-CSP and hydrogenated-R-POTPHDOCD-CSP were pumped into two chromatographic columns of 250mm×4.6mm (i.d.) specifications by homogenization method respectively, and were mixed in polar organic mode (acetonitrile / methanol / acetic acid / triethylamine) to measure the chromatographic properties of the two columns; the mobile phase was filtered through a 0.45 μm organic microporous membrane before use, and vacuum degassed for 30 minutes; the sample was dissolved in chromatographically pure methanol and filtered through a 0.45 μm needle filter ; The mobile phase flow rate is 0.6mL / min; The detection slope length is 254nm. See attached for separation data Figure 5 .
PUM
 Login to View More
Login to View More Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com