Cyclopoly(N-vinylcaprolactam) as well as preparation method and application thereof
A vinyl caprolactam, cyclic technology, applied in cyclic poly(N-vinyl caprolactam) and its preparation and application fields, can solve the problems such as no cyclic polyresearch reports
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
Embodiment 1
[0048] Add 10 mL of dichloromethane, propynyl alcohol (2.0 mL, 33.7 mmol) and triethylamine (TEA, 5.6 mL, 37.1 mmol) sequentially into a 50 mL round bottom flask, stir and cool to 0 °C in an ice-water bath, and put The dichloromethane solution of 2-chloropropionyl chloride (3.4 mL, 33.8 mL) was slowly dropped into the above solution, stirred at 0°C for 30 min, raised to room temperature, continued stirring for 12 h, filtered, and the filtrate was washed with saturated sodium bicarbonate solution Washed 3 times, dried over anhydrous magnesium sulfate, filtered, and evaporated the solvent to obtain a light yellow transparent liquid, which was separated by silica gel column chromatography (petroleum ether: ethyl acetate = 50: 1) to obtain the atom transfer free Based polymerization (ATRP) initiator (alkynyl-Cl), yield 60.3%.
Embodiment 2
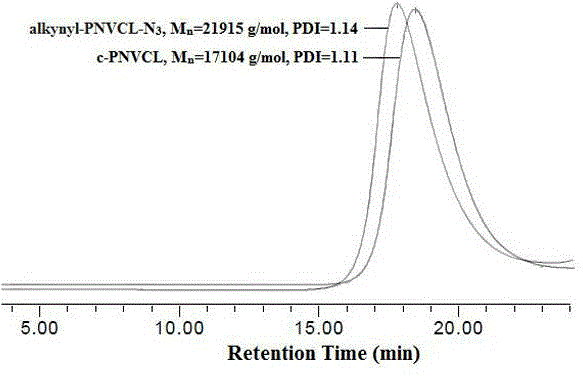
[0050] Add NVCL (0.6103 g, 4.38 mmol) into a 10 mL polymerization tube, vacuumize and fill with nitrogen several times, then add CuCl (0.0039 g, 0.040 mmol), CuCl 2 (0.0006 g, 0.004 mmol), Me 6 Mixed solvent of Cyclam (0.0125 g, 0.044 mmol) and 0.2 mL of dioxane / isopropanol, continue vacuuming and nitrogen filling for 30 min, add alkynyl initiator alkynyl-Cl (0.0006 g, 0.0044 mmol), and react at 30°C After 2 h, the product was dissolved in distilled water, and the insoluble matter was removed by filtration. The filtrate was dialyzed in a dialysis bag with a molecular weight cut off of 3500 for 6 days, concentrated, and dried in vacuum for 24 h to obtain PNVCL (alkynyl-PNVCL-Cl) with an alkynyl group at the end.
Embodiment 3
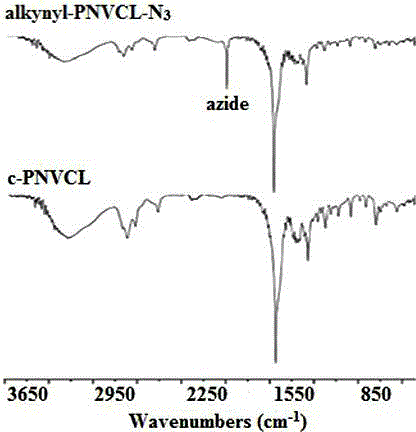
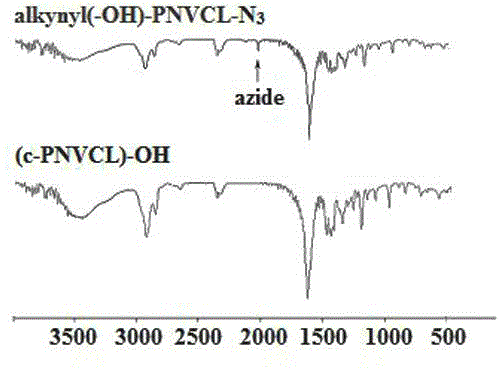
[0052] Add 8.5 g of PNVCL (alkynyl-PNVCL-Cl) with an alkynyl group in a 100 mL round bottom flask, 30 mL of DMF, and after the polymer is dissolved, add 0.33 g of NaN 3 , the reaction mixture was stirred at 45°C for 48 h, DMF was distilled off under reduced pressure, the product was dissolved in THF, separated by alumina column chromatography (dichloromethane was the eluent), concentrated by distillation, and dried in vacuum for 24 h to obtain Poly(N-vinylcaprolactam) (alkynyl-PNVCL-N) with alkynyl and azido groups at both ends 3 ), the yield was 93%.
PUM
 Login to View More
Login to View More Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com