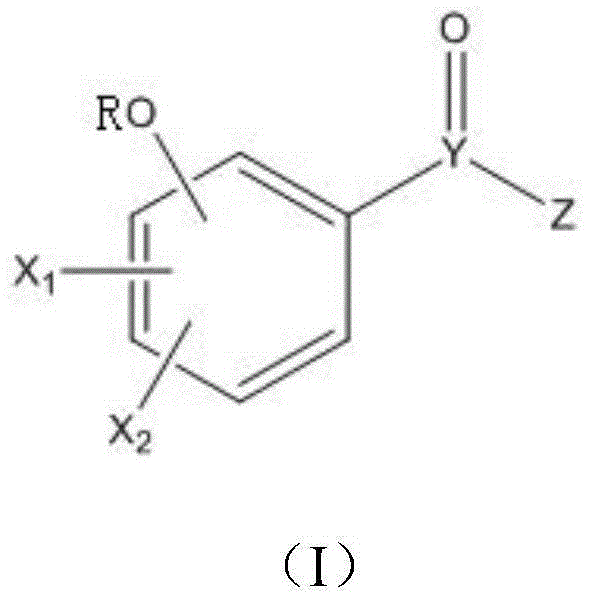
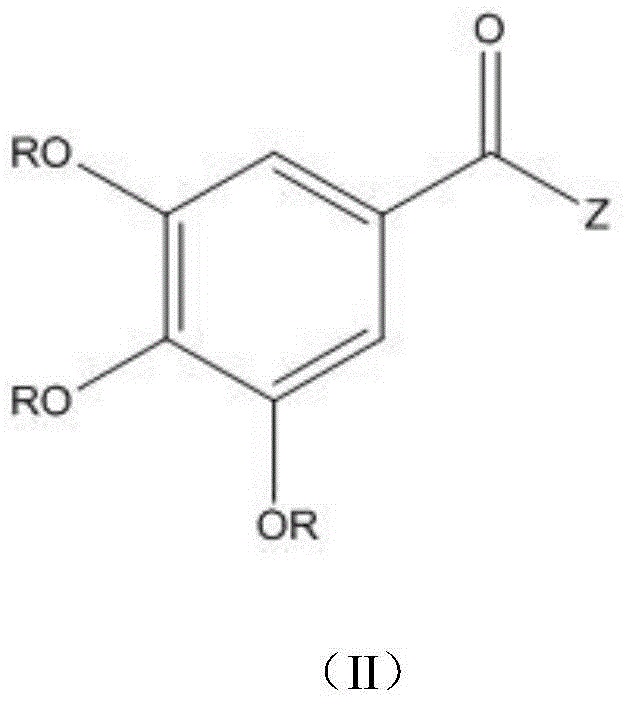
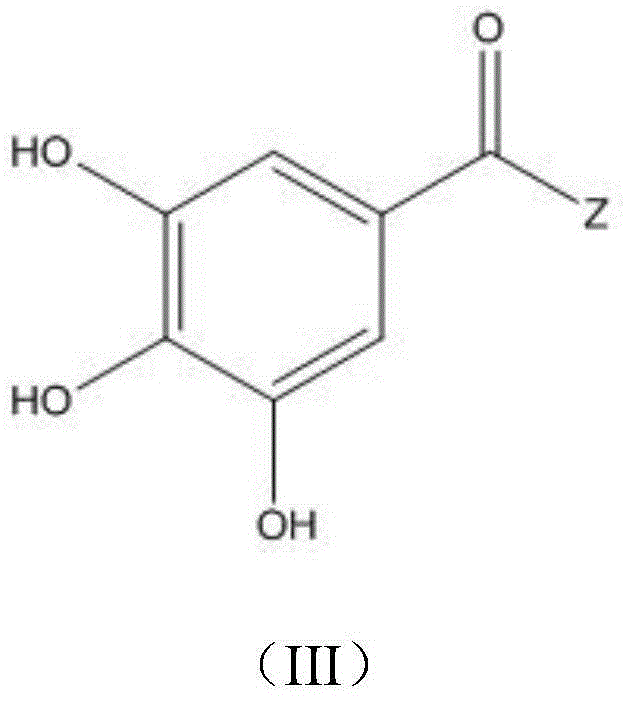
Application of gallate derivative to preparation of medicine for treating hyperuricemia
A technology of gallic acid ester and hyperuricemia, applied in the field of chemical medicine, can solve the problems of no further animal experimental data, triggering acute gout attack, and large toxic and side effects of drugs, achieving no toxic and side effects, reducing serum uric acid level, and safety. high sex effect
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
Embodiment 1
[0079] Example 1: Extraction and characterization of compound 1-3 of the present invention
[0080] Take 5 kg of emblica medicinal material, pulverize it, and extract it with 5 L of water for 40 min while keeping it slightly boiling. The extract was concentrated to 1 L in vacuo, and the concentrated solution was separated by column chromatography using Diaion HP-20 macroporous adsorption resin, and eluted with water, 30% ethanol, 60% ethanol, and 95% ethanol respectively. Take 20 g of the eluent eluted with water and use reverse phase C18 silica gel for column chromatography separation, and use 5%-10% ethanol as the eluent for elution to obtain compound 1, compound 2 and compound 3 of the present invention respectively ; The detection and characterization data are as follows:
[0081] Compound 1: C 11 h 10 o 9 , molecular weight 287[M+H] +
[0082] 1 H-NMR(acetone-d6,300MHz)δ:3.03(1H,d),3.06(1H,d),5.60(1H,dd),7.17(2H,s,); 13 C-NMR (acetone-d6, 75 MHz) δ: 170.9, 170.6, ...
Embodiment 2
[0087] Embodiment 2: Preparation and characterization of compound 4 of the present invention
[0088] In this example, Compound 4 of the present invention was prepared using the following synthetic route:
[0089]
[0090] The specific operation is as follows:
[0091] (1) Synthesis of 3,4,5-tribenzylbenzoic acid
[0092] Dissolve 17.0 g of gallic acid in 800 ml of dimethylformamide (abbreviated as DMF, the same below), and use nitrogen protection, add 113 g of anhydrous potassium carbonate in batches at 25 ° C, stir for 1 hour, heat up to 40 ° C, and then Add 143ml of benzyl bromide dropwise to the reaction system within 30min; after the dropwise addition, stir the reaction mixture at 40°C for 12h; use TLC to confirm that the reaction is complete, stop heating, and lower the reaction temperature to Room temperature 25°C; add 400ml of water and 1L of ethyl acetate to the reaction system, separate liquid extraction, wash the organic phase with 500ml of water three times, t...
Embodiment 3
[0102] Embodiment 3: Preparation and characterization of compound 5 of the present invention
[0103] In this example, compound 5 of the present invention was prepared using the same synthetic route and method as compound 4, the only difference being that the n-butanol in step 3) was replaced with 3-hydroxymethyl-benzoic acid to obtain this compound Compound 5 was invented, and the product was detected and characterized as follows:
[0104] Compound 5: C 15 h 12 o 7 , molecular weight 305[M+H] +
[0105] 1 H NMR (400MHz, CD 3 OD)δ8.14(s,1H),8.05(s,1H),7.81(d,1H),7.45(t,1H),6.95(s,2H),5.26(s,2H); 13 C NMR (100MHz, CD 3 OD) δ169.3, 165.9, 146.1.146.1, 141.1, 140.3, 132.3, 130.4, 129.2, 128.8, 128.6, 121.2, 109.6, 109.6, 65.7. It can be seen that its structure is correct.
PUM
 Login to View More
Login to View More Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com