Canine interferon α particle complex and its preparation method and application
A technology of canine interferon and α particles, which is applied in the direction of pharmaceutical formulas, medical preparations with non-active ingredients, medical preparations containing active ingredients, etc., to achieve the effects of good biocompatibility, slow down degradation, and mild preparation conditions
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
Embodiment 1P
[0030] Example 1 Synthesis of PGA-PAE material
[0031] Weigh 0.1 g of γ-polyglutamic acid and dissolve it in 5 ml of pure water to prepare a 2% PGA solution. The pH of the PGA solution was adjusted to 3.0 with 1 mol / L hydrochloric acid. The PGA solution was put into a 98°C water bath for degradation for 11 min, and the temperature was cooled in an ice bath immediately after the degradation. The pH was then adjusted to 7.0 with 1 mol / L sodium hydroxide solution. 0.297 g of EDC.I and 0.146 g of ethyl phenylalanine were added sequentially. The reaction was carried out on a shaker at 37°C and 210rpm for 24h. After the reaction, the reaction system was centrifuged at 12,000 rpm for 1 min, and the precipitate was resuspended with pure water. Repeat centrifugation 2 times. Dry the white precipitate. Weighing, dissolving the precipitate with dimethyl sulfoxide, and making up to 40 mg / ml, the PGA-PAE material is obtained.
Embodiment 2
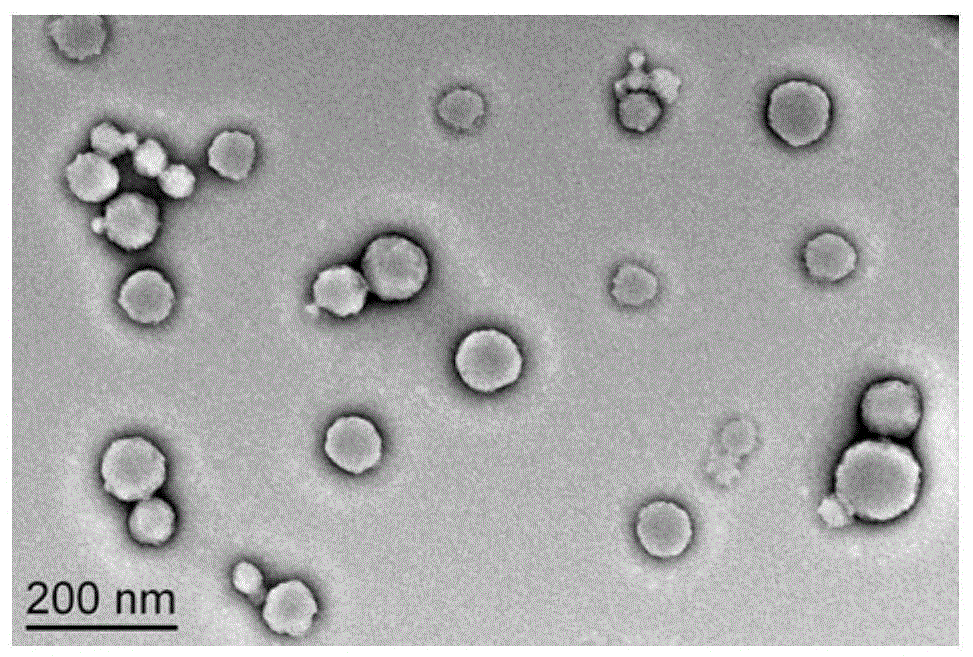
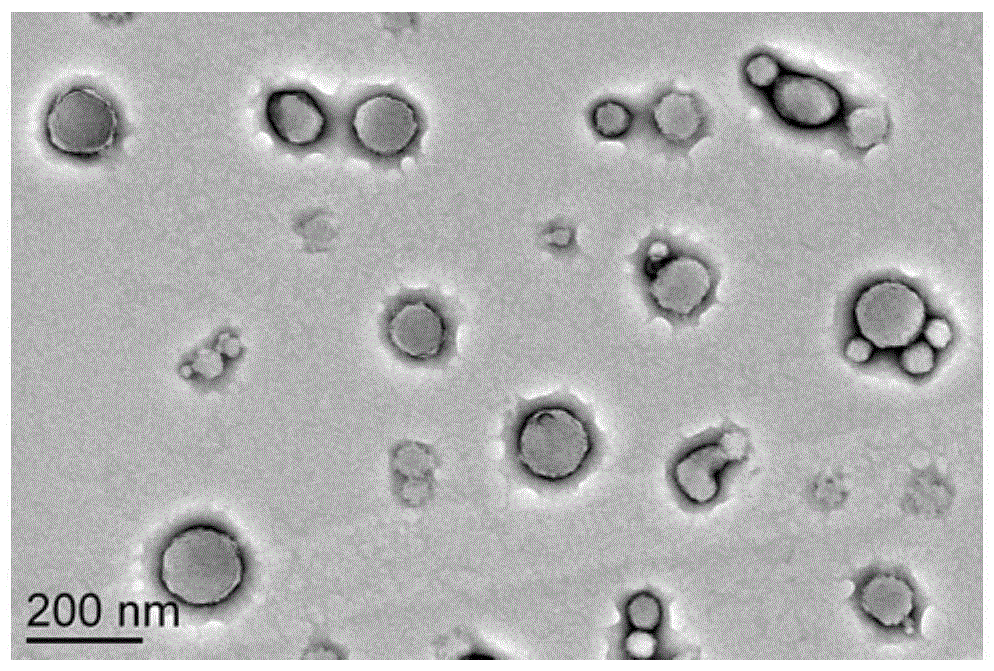
[0032] Example 2 Preparation of canine interferon alpha particle complex CaIFNα-NPs encapsulated and fused with canine interferon alpha
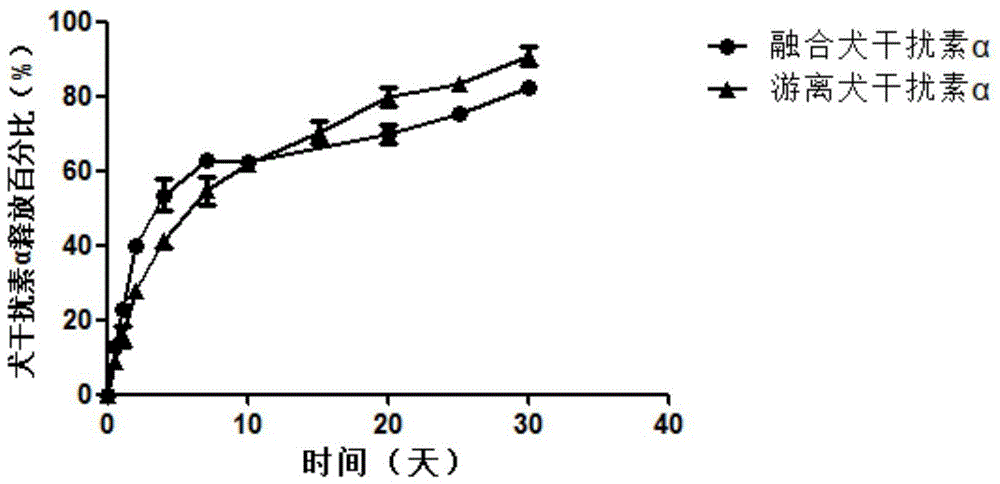
[0033] Take 1ml of the above PGA-PAE material, slowly drop it into 1ml of 0.25mg / ml fused canine interferon alpha aqueous solution, and mix to obtain a white emulsion. The emulsion was centrifuged at 16000 rpm for 15 min. The pellet was separated from the supernatant. The pellet was resuspended with 2 ml of pure water and centrifuged again at 16000 rpm for 15 min. The precipitate was separated from the supernatant, and the precipitate was lyophilized to obtain CaIFNα-NPs.
[0034] Wherein, the sequence of the fusion canine interferon alpha is shown in SEQ ID No: 1.
[0035] In order to verify whether the fusion canine interferon alpha was encapsulated into the particles and to calculate the encapsulation rate of the fusion canine interferon alpha, CaIFNα-NPs were cleaved with 4% sodium dodecyl sulfonate solution. Since CaIFNα-NPs are for...
Embodiment 3
[0039] Example 3 Preparation of canine interferon alpha particle complex CaIFNα-NPs encapsulating free canine interferon alpha
[0040] Take 1 ml of the above PGA-PAE material, slowly drop it into 1 ml of 0.4 mg / ml free canine interferon alpha aqueous solution, and mix to obtain a white emulsion. The emulsion was centrifuged at 16000 rpm for 15 min. The pellet was separated from the supernatant. The pellet was resuspended with 2 ml of pure water and centrifuged again at 16000 rpm for 15 min. The precipitate was separated from the supernatant, and the precipitate was lyophilized to obtain CaIFNα-NPs.
[0041] Wherein, the sequence of free canine interferon alpha is shown in SEQ ID No:2.
[0042] To verify whether free canine interferon alpha was encapsulated into particles and to calculate the encapsulation rate of free canine interferon alpha, CaIFNα-NPs were cleaved with 4% sodium dodecyl sulfonate solution. Since CaIFNα-NPs are formed by the amphiphilic self-polymerizati...
PUM
| Property | Measurement | Unit |
|---|---|---|
| particle diameter | aaaaa | aaaaa |
| particle diameter | aaaaa | aaaaa |
| particle diameter | aaaaa | aaaaa |
Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com