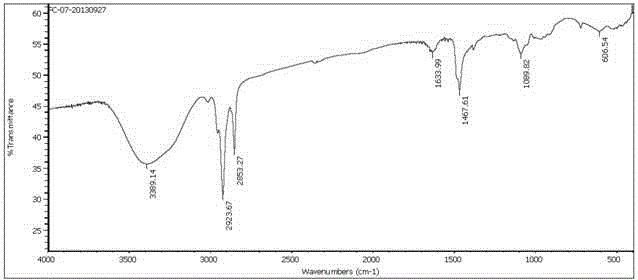
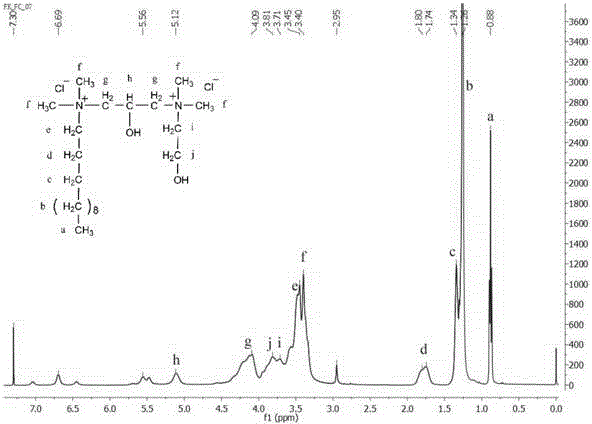
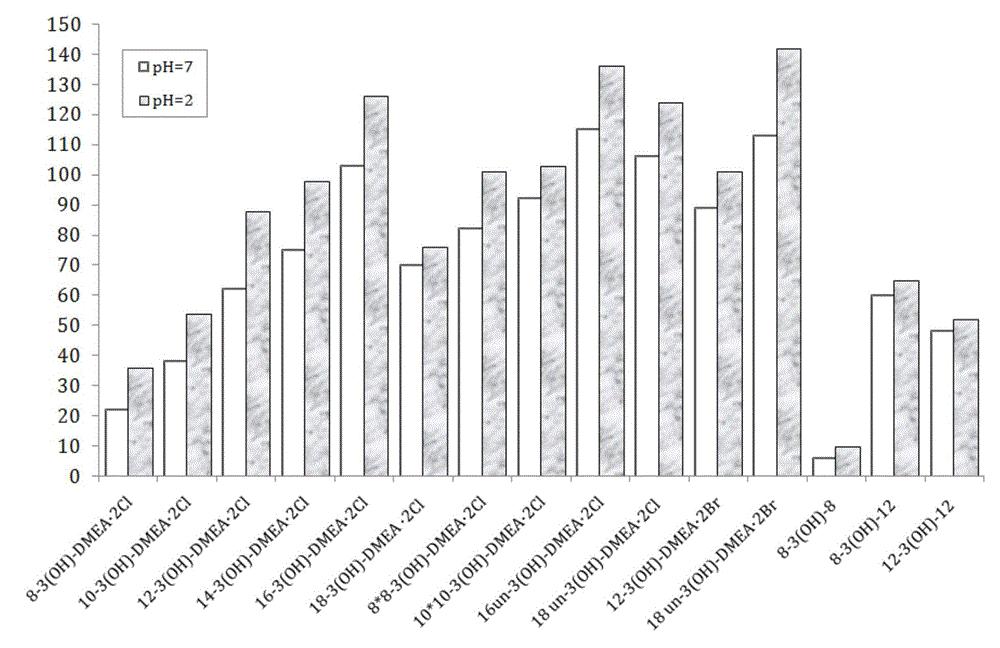
Dissymmetric cationic Gemini surfactant and preparation method thereof
A Gemini surface, cationic technology, applied in chemical instruments and methods, preparation of organic compounds, preparation of amino hydroxyl compounds, etc., can solve the problems of poor solubility, high viscosity of aqueous solution, poor acid resistance and alkali resistance, etc., to achieve easy reaction, amine Low content, reducing the corrosion effect of equipment
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
Embodiment 1
[0081] Example 1: Preparation of 12-3(OH)-DMEA 2Cl asymmetric cationic Gemini surfactant
[0082] The first step: preparation of tertiary amine hydrochloride intermediate
[0083] In a 500ml four-necked flask equipped with a stirring device and a condensing reflux device, add 115.76 g of aqueous hydrochloric acid solution prepared in advance, containing 0.19 mol of HCl; (1) Add dropwise into the HCl aqueous solution that has been warmed up to 30° C. in advance, and maintain the temperature during the dropwise addition reaction. The rate of addition is subject to the fact that a large amount of white flocs will not gather in the hydrochloric acid aqueous solution. After the dropwise addition, keep at 30° C. and continue stirring for 0.5 h to obtain an aqueous solution of dodecyldimethyl tertiary amine hydrochloride intermediate (II). At this time, the tertiary amine hydrochloride accounts for 30% of the total mass fraction of the reaction system.
[0084] The second step: pr...
Embodiment 2
[0091] Example 2: Preparation 18 un -3(OH)-DMEA·2Cl Asymmetric Cationic Gemini Surfactant
[0092] The first step: preparation of tertiary amine hydrochloride intermediate
[0093] In a 500ml four-neck flask equipped with a stirring device and a condensing reflux device, add 199.23 g of a pre-prepared aqueous hydrochloric acid solution containing 0.2 mol of HCl; The amine (I) was added dropwise to the aqueous HCl solution warmed to 50°C in advance, and the temperature was maintained during the dropwise addition reaction. The rate of addition is subject to the fact that a large amount of white flocs will not gather in the hydrochloric acid aqueous solution. After the dropwise addition, keep at 50° C. and continue stirring for 0.5 h to obtain an aqueous solution of 9-octadecenyl dimethyl tertiary amine hydrochloride intermediate (II). At this time, the tertiary amine hydrochloride accounts for 25% of the total mass fraction of the reaction system.
[0094] The second step: p...
Embodiment 3
[0110] Example 3: Preparation of 8-3(OH)-DMEA 2Cl asymmetric cationic Gemini surfactant
[0111] The first step: preparation of tertiary amine hydrochloride intermediate
[0112] In the 500ml four-necked flask that stirring device, condensing reflux device are housed, add the hydrochloric acid aqueous solution 75.33g that prepares in advance, wherein contain HCl 0.34mol; ) was added dropwise into 20° C. HCl aqueous solution, and the temperature was maintained during the dropwise reaction. The rate of addition is subject to the fact that a large amount of white flocs will not gather in the hydrochloric acid aqueous solution. After the dropwise addition, keep at 20° C. and continue stirring for 0.5 h to obtain an aqueous solution of octyldimethyl tertiary amine hydrochloride intermediate (II). At this time, the tertiary amine hydrochloride accounts for 50% of the total mass fraction of the reaction system.
[0113] The second step: preparation of 3-chloro-2-hydroxypropyl-quat...
PUM
 Login to View More
Login to View More Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com