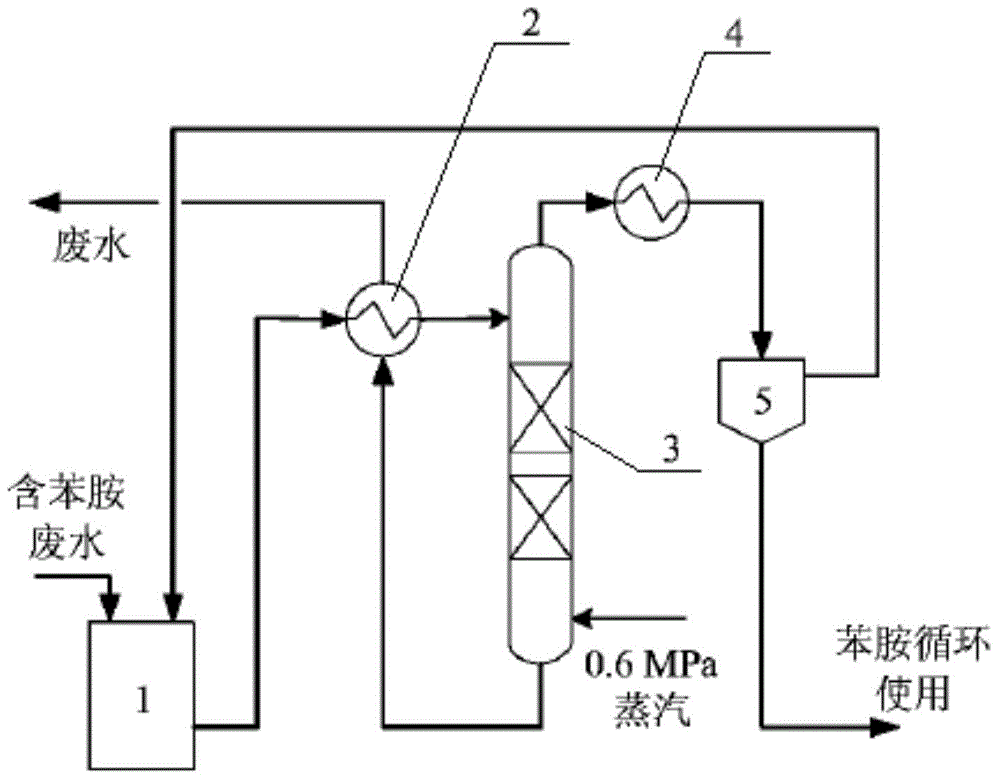
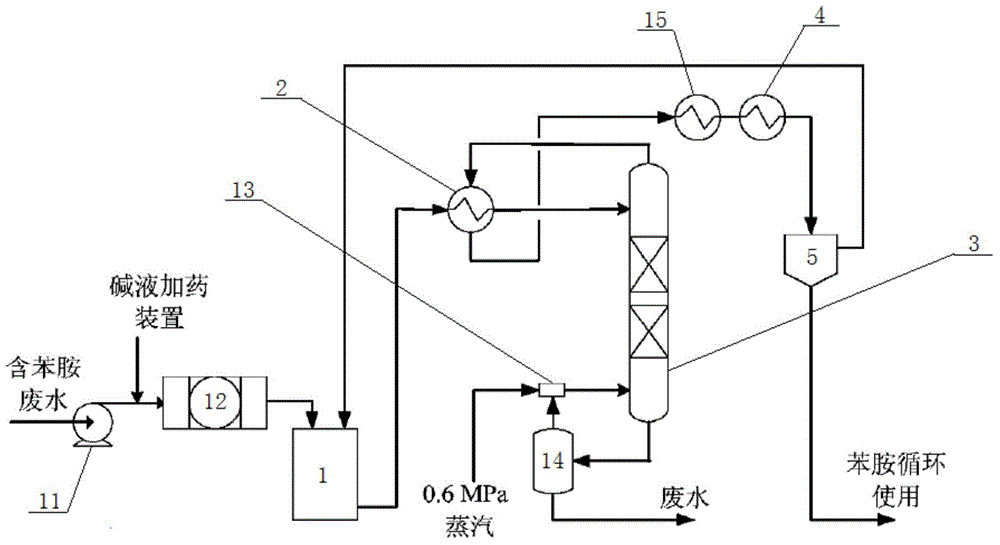
Recycling production process for nigrosine
A production process and nigrosine technology, applied in the field of dyes, can solve the problems of not fully utilizing the latent heat of the gas phase and wasting heat, and achieve the effects of protecting the environment, saving steam costs, and preventing volatilization
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
Embodiment 1
[0060] 1) Aniline and nitrobenzene are carried out condensation reaction in condensation reactor 16 under the condition that catalyst exists, obtain the nigrosine product containing excess aniline, wherein the mass ratio of aniline and nitrobenzene is 4:1, the condensation reaction The temperature is 160°C, the condensation reaction time is 4 hours, and the catalyst is FeCl 3 ;
[0061] 2) In the presence of hydrochloric acid and hot water, the nigrosine product containing excess aniline obtained in step 1) is acidified in the acidification reaction kettle 17, and then the product obtained is centrifuged by a centrifuge 18 to obtain the nigrosine product and the nigrosine product containing Aniline wastewater, wherein the reaction temperature of the acidification reaction is 90°C, and the acidification reaction time is 1 hour;
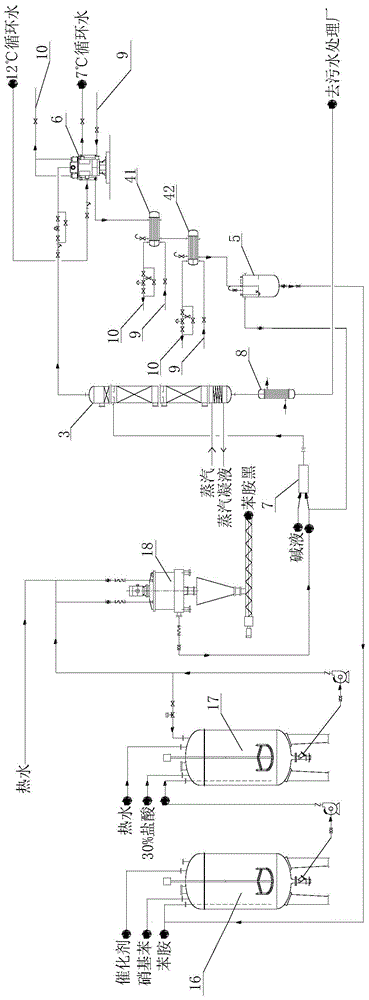
[0062] 3) if image 3 As shown, the lye and the aniline-containing wastewater with an aniline content of 4wt% are mixed by the static mixer 7, and t...
Embodiment 2
[0067] 1) Aniline and nitrobenzene are carried out condensation reaction in condensation reactor 16 under the condition that catalyst exists, obtain the nigrosine product containing excess aniline, wherein the mass ratio of aniline and nitrobenzene is 6:1, the condensation reaction The temperature is 200°C, the condensation reaction time is 6 hours, and the catalyst is FeCl 3 ;
[0068] 2) In the presence of hydrochloric acid and hot water, the nigrosine product containing excess aniline obtained in step 1) is acidified in the acidification reaction kettle 17, and then the product obtained is centrifuged by a centrifuge 18 to obtain the nigrosine product and the nigrosine product containing Aniline wastewater, wherein the reaction temperature of the acidification reaction is 100°C, and the acidification reaction time is 2 hours;
[0069] 3) if image 3 As shown, after the lye and the aniline-containing wastewater with an aniline content of 3wt% are mixed by the static mixer ...
Embodiment 3
[0074] 1) Aniline and nitrobenzene are carried out condensation reaction in condensation reactor 16 under the condition that catalyst exists, obtain the nigrosine product containing excess aniline, wherein the mass ratio of aniline and nitrobenzene is 5:1, the condensation reaction The temperature is 180°C, the condensation reaction time is 5 hours, and the catalyst is FeCl 3 ;
[0075]2) In the presence of hydrochloric acid and hot water, the nigrosine product containing excess aniline obtained in step 1) is acidified in the acidification reaction kettle 17, and then the product obtained is centrifuged by a centrifuge 18 to obtain the nigrosine product and the nigrosine product containing Aniline wastewater, wherein the reaction temperature of the acidification reaction is 95°C, and the acidification reaction time is 1.5 hours;
[0076] 3) if image 3 As shown, the lye and the aniline-containing wastewater with an aniline content of 8wt% are mixed by the static mixer 7, and...
PUM
 Login to View More
Login to View More Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com