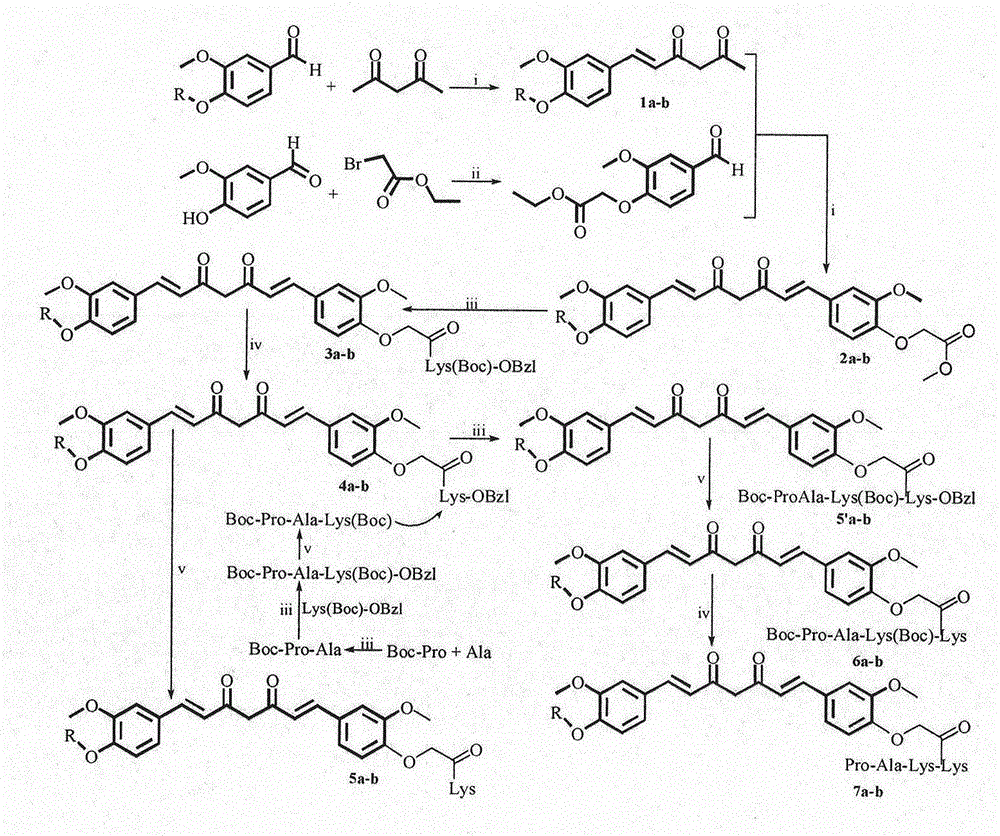
Lys(Pro-Ala-Lys) curcumin derivatives, synthesis thereof and application thereof in medical science
A technology of drugs and anti-inflammatory drugs, applied in the field of biomedicine, can solve the problem that curcumin does not have thrombolytic activity and other problems
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
Embodiment 1
[0033] Embodiment 1 prepares Boc-Pro-Ala-Lys (Boc)
[0034] 1) Preparation of Boc-Pro-Ala
[0035] 1.075g (5.0mmol) of Boc-Pro was dissolved in 20mL of anhydrous THF, and 0.637g (5mmol) of N-hydroxysuccinimide (HOSu) was added to the solution under ice cooling, and completely dissolved. Under ice-cooling, 1.236 g (6.0 mmol) of dicyclohexylcarbodiimide (DCC) dissolved in a small amount of anhydrous THF was added to the reaction solution. Stir at room temperature for 7 h, and monitor the disappearance of Boc-Pro by TLC (petroleum ether / ethyl acetate, 3:1). Dicyclohexylurea (DCU) was filtered off, and the filtrate was concentrated under reduced pressure to remove THF. The residue was dissolved with ethyl acetate, and the solution was successively washed with saturated NaHCO 3 After washing with aqueous solution and saturated NaCl aqueous solution, the ethyl acetate layer was concentrated to dryness under reduced pressure, the residue was dissolved by adding an appropriate amou...
Embodiment 2
[0040] Example 2 Preparation of 1-(3-methoxy-4-hydroxyphenyl)-7-[(4-oxoacetoxy)-3-methoxyphenyl]-1,6-heptadiene-3, 5-diketone
[0041] 1) Preparation of 6-(3-methoxy-4-hydroxyphenyl)-5,6-hexene-2,4-dione
[0042] Put 31mL (0.3mol) of acetylacetone in a 250mL three-neck flask, add 14.5g (0.21mol) of boron trioxide and 70mL of ethyl acetate, react at 70°C for one hour to turn into a white suspension, then add 29.5mL ( 0.11mol) tri-n-butyl borate and 15.2g (0.1mol) 3-methoxy-4-hydroxybenzaldehyde, react at 85°C for 0.5 hours to make the solution a light yellow suspension, add 10.88mL (0.11 mol) ethyl acetate solution of n-butylamine, react for 1 hour to make the solution turn red, cool down to 50°C, add 200mL 1N hydrochloric acid, react for 0.5 hour, stop the reaction. The aqueous layer was separated, extracted three times with 40 mL ethyl acetate, combined ethyl acetate, dried with anhydrous sodium sulfate, filtered off sodium sulfate, the filtrate was desolvated under reduced...
Embodiment 3
[0048] Example 3 Preparation of 1-(3,4-di-methoxyphenyl)-7-[(4-oxoacetoxy)-3-methoxyphenyl]-1,6-heptadiene-3,5 - dione
[0049] 1) Preparation of 6-(3,4-di-methoxyphenyl)-5,6-hexene-2,4-dione
[0050] According to the preparation method of item 1) in Example 2, 16.6g (0.1mol) 3,4-di-methoxybenzaldehyde and 31mL (0.3mol) acetylacetone were reacted to obtain 8.6g (34.6% ) the title compound as a pale yellow solid. ESI-MS(m / e): 235[M+H] + .
[0051] 2) 1-(3,4-di-methoxyphenyl)-7-(4-oxoacetylethyl-3-methoxyphenyl)-1,6-heptadiene-3,5-dione preparation of
[0052] According to the preparation method of item 1) in Example 2, by 1.097g (4.42mmol) 6-(3,4-di-methoxyphenyl)-5,6-hexene-2,4-diketone and 1.088g ( 4.87 mmol) of 3-methoxy-4-oxoacetoethoxybenzaldehyde was reacted and purified by silica gel column chromatography to obtain 0.435 g (21.0%) of the title compound as a pale yellow solid. ESI-MS(m / e): 469.6[M+H] + .
[0053] 3) 1-(3,4-di-methoxyphenyl)-7-(4-oxoacetoxy-3-meth...
PUM
| Property | Measurement | Unit |
|---|---|---|
| The inside diameter of | aaaaa | aaaaa |
| The inside diameter of | aaaaa | aaaaa |
| Outer diameter | aaaaa | aaaaa |
Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com