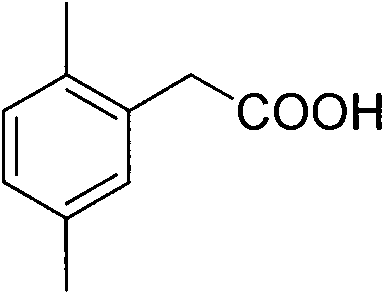
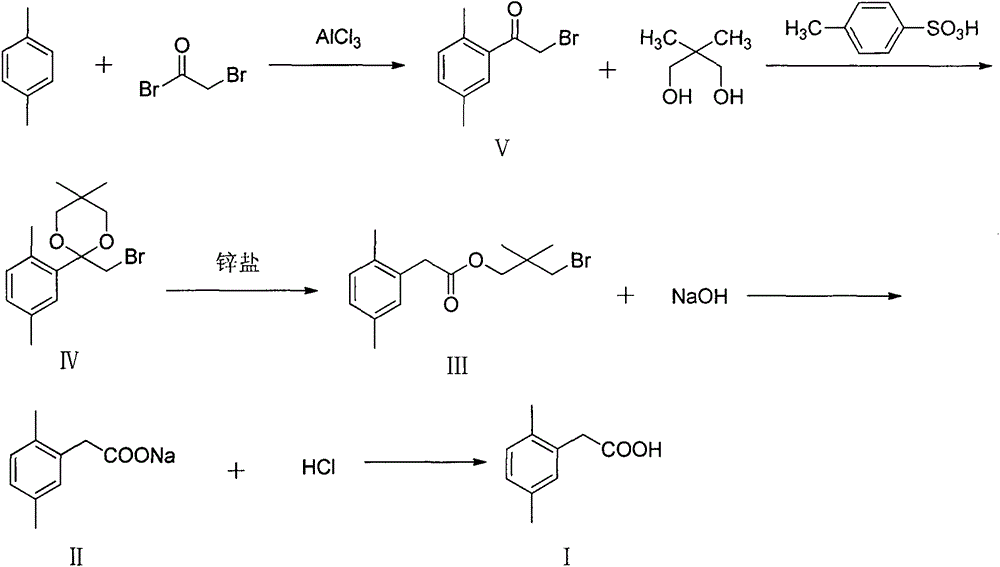
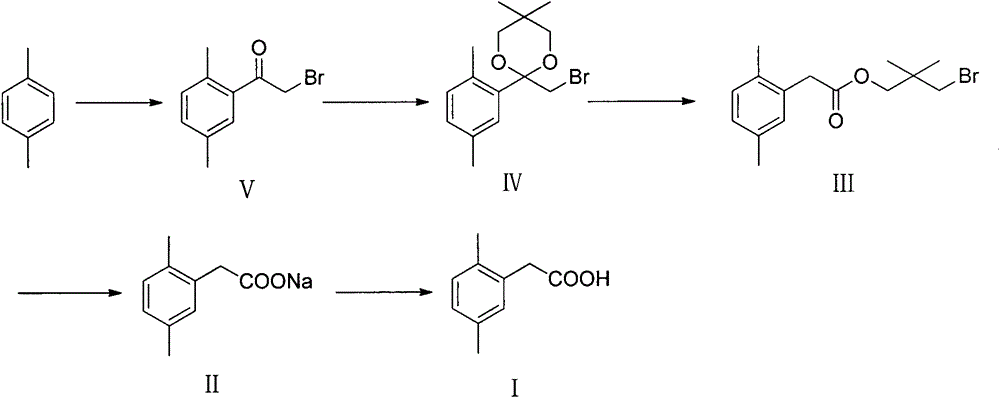
Preparation method of 2,5-dimethylphenylacetic acid
A technology of dimethylphenylacetic acid and dimethylphenyl, applied in the field of preparing spirotetramat key intermediate 2, can solve the problems of inability to carry out industrialized production, no industrialization significance, a large amount of sulfur-containing wastewater, waste residue and the like
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
Embodiment 1
[0046] Synthesis of compound (V)
[0047]
[0048] In a 250ml reaction flask, add 120ml of dichloroethane, cool to -5~0°C, add 21.23g (0.20mol) of p-xylene, 34.67g (0.26mol) of anhydrous aluminum trichloride, stir, and dropwise add bromine 48.44 g (0.24 mol) of acetyl bromide, after the dropwise addition, it was raised to room temperature for reaction until the reaction was completed. The product was poured into 100ml of 5% ice-hydrochloric acid solution, stirred for 30min, separated, the aqueous layer was extracted with dichloroethane, the organic phases were combined, washed with water, and the solvent was removed to obtain 41.97g of a yellow liquid product with a yield of 92.41%.
[0049] 1 H NMR (CDCl 3 , 300Hz) δ: 2.35 (s, 3H, CH 3 ), 2.50(s, 3H, CH3), 4.43(s, 2H, CH2), 7.14~7.24(m, 2H, Ar-H), 7.45(s, 1H, Ar-H); ESI-MS: 251.0[ M+Na] + .
Embodiment 2
[0051] Synthesis of compound (IV)
[0052]
[0053] In a 500ml reaction flask, add 34.06g (0.15mol) of 2,5-dimethylbromoacetophenone, 15.62g (0.15mol) of neopentyl glycol, a catalytic amount of p-toluenesulfonic acid, 200ml of toluene, and heat to 110-115°C Reflux with water until the raw materials react completely to obtain yellow 2-bromomethyl-2-(2,5-dimethylphenyl)-5,5-dimethyl-[1,3]dioxane The toluene solution of alkane, wherein the product is 42.32g (GC analysis), the yield is 90.09%.
[0054] 1 H NMR (CDCl3 , 300Hz) δ: 0.62 (s, 3H, CH 3 ), 1.39(s, 3H, CH3), 2.35(m, 6H, CH3), 3.47(m, 6H, CH3), 7.07(s, 2H, Ar-H), 7.30(s, 1H, Ar-H) ;ESI-MS: 335.1[M+Na] + .
Embodiment 3
[0056] Synthesis of compound (III)
[0057]
[0058] The toluene solution of 2-bromomethyl-2-(2,5-dimethylphenyl)-5,5-dimethyl-[1,3]dioxane obtained in the previous step was cooled to room temperature, Add ZnCl 2 4.09g (0.03mol), warming up to reflux, monitoring the reaction process to the end, to obtain 3-bromo-2,2-dimethylpropyl-2-(2,5 dimethylphenyl) acetate (III) Toluene solution, wherein product 36.44g (GC analysis), yield 86.10%.
[0059] 1 H NMR (CDCl 3 , 300Hz) δ: 0.83~1.25(m, 6H, CH 3 ), 2.27(m, 3H, CH3), 2.47(m, 3H, CH3), 3.20(s, 2H, CH2), 3.61(s, 2H, CH2), 3.94(s, 2H, CH2), 7.04(m , 3H, Ar-H); ESI-MS: 335.0[M+Na] + .
PUM
 Login to View More
Login to View More Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com