Preparation method of Vortioxetine
A technology of dimethylphenylthio group and cyclization reaction, applied in directions such as organic chemistry, can solve the problems of unsuitability for large-scale production, low yield and high cost, and achieve the effects of no toxic side effects, common reagents, and cost reduction
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
Embodiment 1
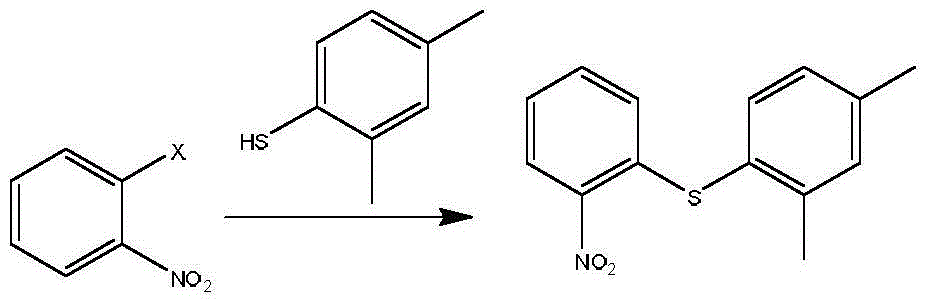
[0021] Example 1: Preparation of 2-(2,4-dimethylphenylthio)nitrobenzene
[0022] 5.1g (36.2mmol) of o-fluoronitrobenzene, 5.0g (36.2mmol) of 2,4-dimethylthiophenol, and 3.0g (21.7mmol) of potassium carbonate were stirred in DMF (30ml) at 80°C for 3h until the reaction was complete , add water (50ml), extract with ethyl acetate (30ml×3), combine the organic layers, wash with brine, dry, concentrate to dryness, add petroleum ether (40ml), stir, filter, wash with petroleum ether, and dry 8.3g of yellow solid , yield 88.6%.
[0023] MS(m / z):260.08[M+H] + ; 1 H-NMR (400MH Z , CDCl 3 )δ:8.26(dd,J 1 =1.2H Z ,J 2 =8.4H Z ,1H),7.49(d,J=8Hz,1H),7.31(m,1H),7.20(m,2H),7.13(d,J=7.6Hz,1H),6.74(dd,J 1 =1.2Hz,J 2 =8.4Hz,1H),2.42(s,3H),2.33(s,3H).
Embodiment 2-4
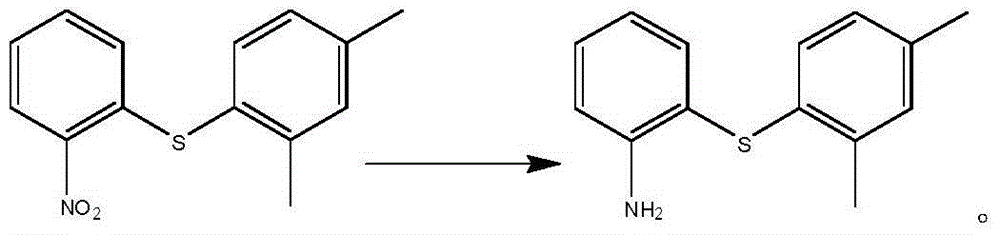
[0024] Example 2-4: Preparation of 2-(2,4-dimethylphenylthio)aniline
[0025] 8.0 g (30.8 mmol) of the compound obtained in Example 1, 10% Pd / CO 0.8 g, and 40 ml of ethanol were hydrogenated at 50 °C for 12 h at normal pressure until the reaction was complete, filtered, washed with ethanol, and concentrated to dryness to obtain 7.0 g of oil. MS(m / z):230.11[M+H] + ; 1 H-NMR (400MH Z , CDCl 3 )δ:7.37(dd,J 1 =1.6Hz,J 2 =8Hz,1H),7.28(s,1H),7.23(m,1H),6.88(dd,J 1 =0.8Hz,J 2 =8Hz,1H),6.83(dd,J 1 =1.2Hz,J 2 =8Hz,1H),6.79(m,2H),4.09(bs,2H),2.42(s,3H),2.29(s,3H).
[0026] 1.0g (3.9mmol) of the compound obtained in Example 1, 1g of iron powder, and 20ml of acetic acid were stirred and reacted at 40°C. After 5 hours, the reaction was complete. Add 30ml of water, extract twice with 50ml of ethyl acetate, and combine the ethyl acetate layer, washed once with saturated brine, washed three times with 30ml 5% NaOH solution, washed twice with brine, dried, filtered, and concentrated ...
Embodiment 5-6
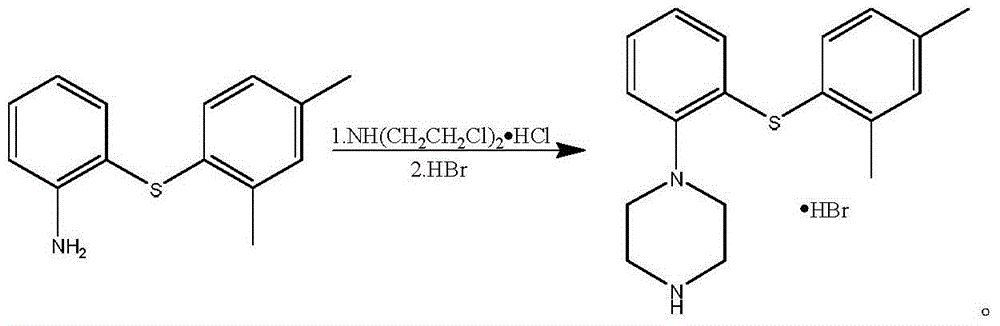
[0028] Example 5-6: Preparation of Vortioxetine·HBr
[0029] Reflux and stir 7.0g of the oil obtained in Example 2, 5.5g (30.8mmol) of bis(2-chloroethyl)amine hydrochloride, and 1,3,5-trimethylbenzene (40ml) overnight, and cool to 60°C , add 80ml of water. Separate the layers, extract the aqueous layer with ethyl acetate (30ml×2), combine the organic layers, wash with brine, dry, and filter. Add 40% hydrobromic acid (about 5ml) dropwise to the filtrate until pH = 1, stir, and wash out the solid. Filter, wash with ethyl acetate (20ml×2), and dry 4.5g of an earth-colored solid.
[0030] Concentrate the filtrate and evaporate ethyl acetate, add 40ml of petroleum ether, stir, pour out the supernatant, add 40ml of petroleum ether, stir and pour out the supernatant. The oil in the lower layer was neutralized with 5% sodium hydroxide solution, extracted with ethyl acetate (30ml×2), the organic layers were combined, washed with brine, dried, filtered, and concentrated to dryness. ...
PUM
 Login to View More
Login to View More Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com