(-)-Swainsonine preparation method
A technology of swainsonine and its synthesis method, which is applied in the direction of organic chemistry, can solve the problems of difficult gram-level products and limited separation, and achieve the effect of simple route, high yield, and simple operation of the technical route
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
Embodiment 1
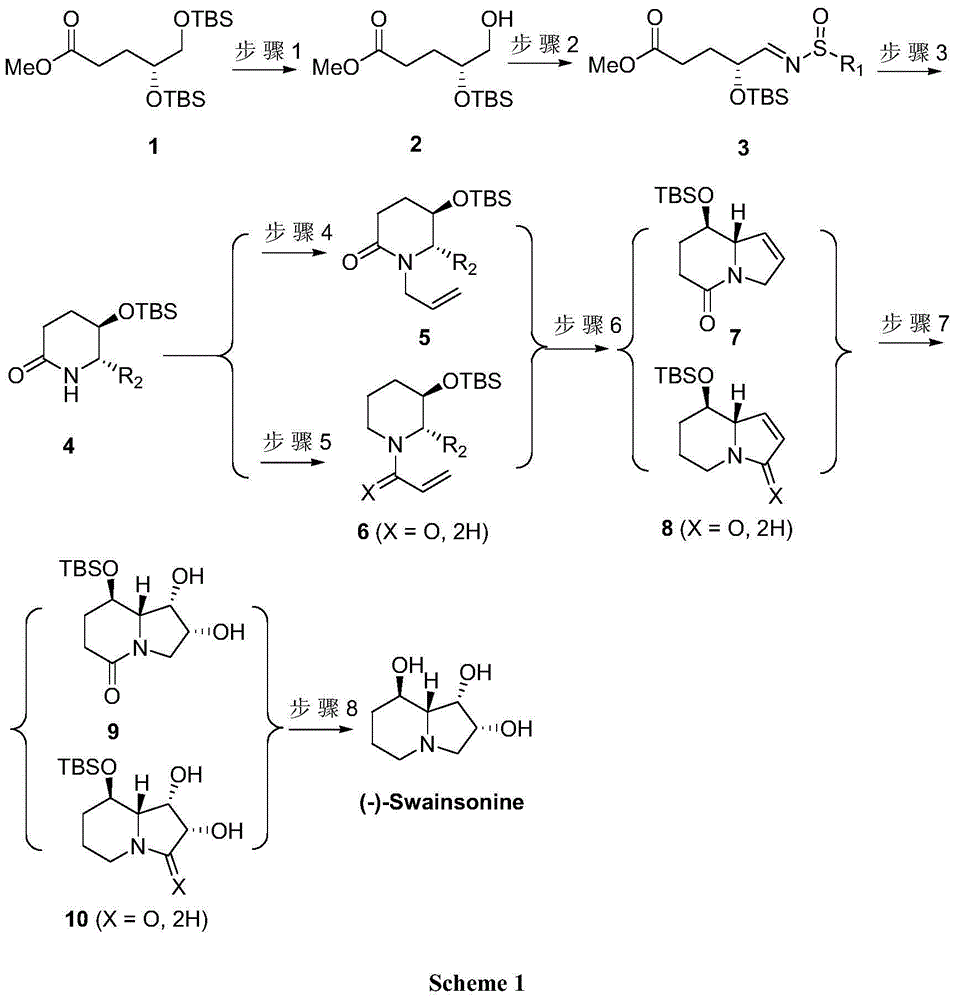
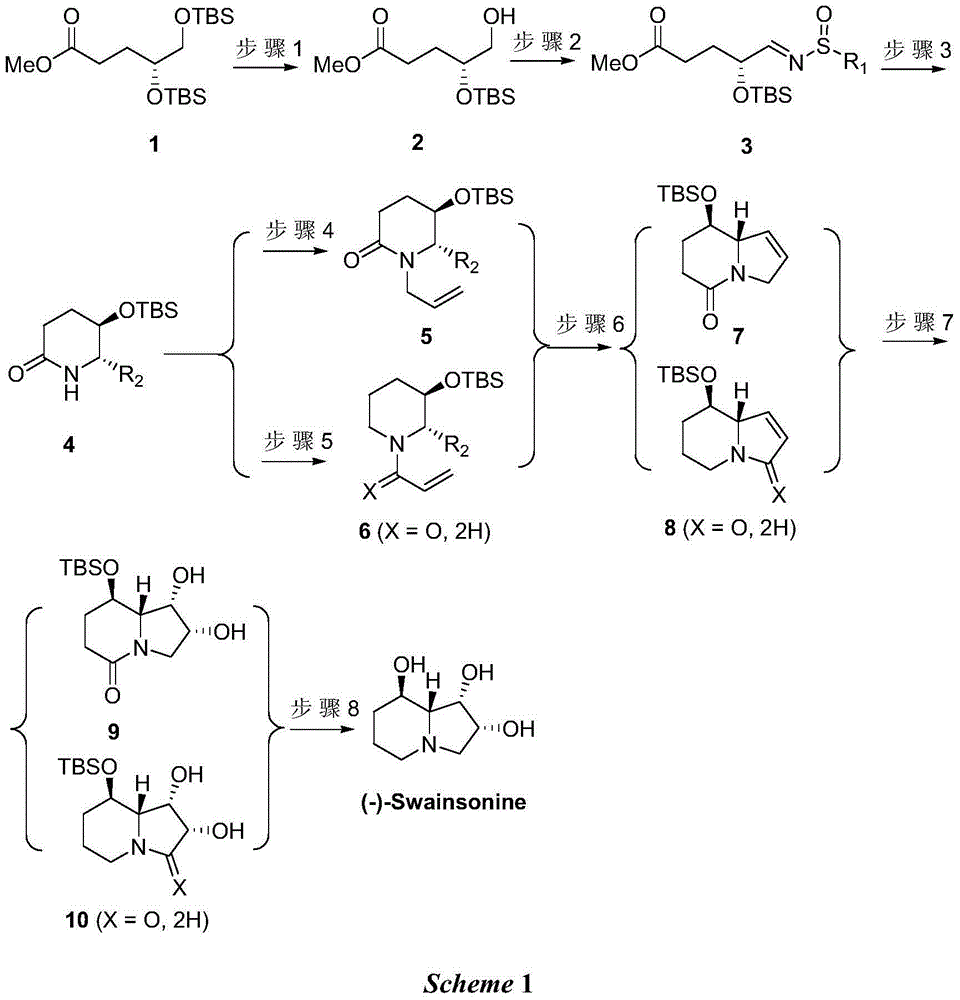
[0021] Synthesis of (R)-methyl 4-(tert-butyldimethylsilyloxy)-5-hydroxyvalerate (2)
[0022] Dissolve compound 1 (10.0g, 26.6mmol) in 100mL of dichloromethane and 80mL of absolute ethanol, cool to -20°C, add camphorsulfonic acid, naturally rise to room temperature and stir for 5 hours, concentrate and filter through silica gel, ethyl acetate After column chromatography of ester and petroleum ether, a colorless liquid 2 was obtained with a yield of 48%.
[0023] 1 HNMR (300MHz, CDCl 3 )δ: 3.86-3.78(m,1H),3.68(s,3H),3.53(dd,J=4.4,11.2Hz,1H),3.47(dd,J=4.8,11.2Hz,1H),2.46-2.32 (m, 2H), 2.08 (brs, 1H), 1.87-1.82 (m, 2H), 0.9 (s, 9H), 0.1 (s, 6H) ppm.
[0024] Synthesis of (R)-4-(tert-butyldimethylsilyloxy)valerate methyl imine (3)
[0025] Compound 2 (5.0g, 19.1mmol) was dissolved in 100mL of dichloromethane, 2 moles of DMP were added at room temperature and stirred for 4 hours, then filtered with a silica gel funnel, directly concentrated and added tert-butylsulfonamide (3.5g)...
Embodiment 2
[0042] The preparation of compounds 2, 3, 5, 7, and 9 was the same as in Example 1.
[0043] Synthesis of (5R,6S)-5-tert-butyldimethylsilyloxy-6-allylcyclohexylamide (4)
[0044] Compound 3 (1.0g, 2.75mmol) and magnesium bromide (5mL, 1MinTHF) were dissolved in 20mL of tetrahydrofuran, and 8.25mL of 1M allylmagnesium bromide was added at -78°C, and then the temperature was naturally raised for 8 hours. After ammonium chloride quenching, ethyl acetate extraction, drying, concentration, silica gel column purification to obtain viscous liquid 4 (R 2 =CH=CH 2; 519mg, 74%).
Embodiment 3
[0046] The preparation of compounds 2, 3 and 4 was the same as in Example 1.
[0047] Synthesis of 1-((2S,3R)-3-(tert-Butyldimethylsilyloxy)-2-vinylpiperidin-1-yl)prop-2-en-1-one (6)
[0048] Compound 4 (R 2 =CH=CH 2; 1.2g, 4.7mmol) and lithium aluminum hydride (357mg, 9.4mmol) were dissolved in 40mL tetrahydrofuran, stirred at 0-50°C for 4 hours, passed through NaSO 4 .10H 2 Quenched by O, filtered and concentrated, dissolved directly in dichloromethane, cooled to -20°C, added acryloyl chloride (846mg, 9.4mmol) and triethylamine (1.4g, 14.1mmol), stirred overnight at natural temperature, and then dissolved in saturated carbonic acid After sodium hydrogen quenching, dichloromethane extraction, concentrated column chromatography purification, to obtain compound 6 (R 2 =CH=CH 2; X=O) 693mg, the yield is 50%.
[0049] The preparation of compounds 8 and 10 (X=O) was the same as that of compounds 7 and 9 in Example 1.
PUM
 Login to View More
Login to View More Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com