A kind of preparation method of plicanatide
A prika, crude peptide technology, applied in the preparation method of peptide, chemical instrument and method, peptide and other directions, can solve the problems of complex composition, method to be improved, difficult separation and purification, etc., to achieve high yield, considerable economy Practical value, low cost effect
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
Embodiment 1
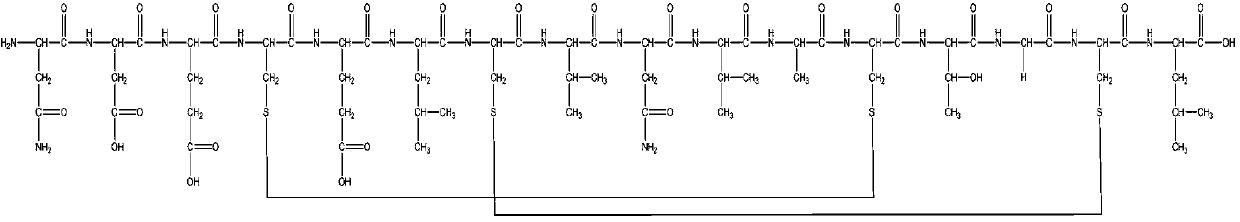
[0044] Weigh 10 g (5.1 mmol) of Fmoc-Leu-king resin with a degree of substitution of 0.51 mmol / g, add it to the solid-phase reactor, wash with DMF 3 times, and swell for 3 h. The piperidine:DMF solution with a volume ratio of 1:4 was added to the reactor for reaction, and after the reaction was washed 2 times with DCM and 4 times with DMF. Fmoc-Cys(Acm)-OH 6.34g, HOBt 2.07g, DIC 2.37mL were weighed and dissolved in DMF solution, mixed evenly, added to the reactor, and reacted at room temperature for 2h. The ninhydrin color reaction control end point, the resin is transparent and colorless, indicating the end of the reaction, if it is colored, continue to react to colorless. After the reaction was completed, DCM was washed twice, and DMF was washed four times.
[0045] Repeat the above steps, deprotect and couple Fmoc-Gly-OH, Fmoc-Thr(tBu)-OH, Fmoc-Cys-(Mmt)-OH, Fmoc-Ala-OH, Fmoc-Val-OH in sequence in sequence , Fmoc-Asn(Trt)-OH, Fmoc-Val-OH, Fmoc-Cys(Acm)-OH, Fmoc-Leu-OH, Fm...
Embodiment 2
[0051] Weigh 10 g (2 mmol) of Fmoc-Leu-king resin with a degree of substitution of 0.2 mmol / g and add it to the solid phase reactor. After washing with DMF 3 times, it swelled for 3 h. The piperidine:DMF solution with a volume ratio of 1:4 was added to the reactor for reaction, and after the reaction was washed 2 times with DCM and 4 times with DMF. Fmoc-Cys(Acm)-OH 1.24g, HOBt 0.406g, DIC 0.465mL were weighed and dissolved in DMF solution, mixed evenly, added to the reactor, and reacted at room temperature for 2h. The ninhydrin color reaction control end point, the resin is transparent and colorless, indicating the end of the reaction, if it is colored, continue to react to colorless. After the reaction was completed, DCM was washed twice, and DMF was washed four times.
[0052] Repeat the above steps, deprotect and couple Fmoc-Gly-OH, Fmoc-Thr(tBu)-OH, Fmoc-Cys-(Mmt)-OH, Fmoc-Ala-OH, Fmoc-Val-OH in sequence in sequence , Fmoc-Asn(Trt)-OH, Fmoc-Val-OH, Fmoc-Cys(Acm)-OH, Fm...
Embodiment 3
[0058] Weigh 10 g (6 mmol) of Fmoc-Leu-king resin with a degree of substitution of 0.6 mmol / g, add it to the solid-phase reactor, wash with DMF 3 times, and swell for 3 h. The piperidine:DMF solution with a volume ratio of 1:4 was added to the reactor for reaction, and after the reaction was washed 2 times with DCM and 4 times with DMF. Weigh 7.46 g of Fmoc-Cys(Acm)-OH, 2.44 g of HOBt, and 2.79 mL of DIC, and dissolve them in DMF solution, mix them evenly, and add them into the reactor, and react at room temperature for 2 h. The ninhydrin color reaction control end point, the resin is transparent and colorless, indicating the end of the reaction, if it is colored, continue to react to colorless. After the reaction was completed, DCM was washed twice, and DMF was washed four times.
[0059] Repeat the above steps, deprotect and couple Fmoc-Gly-OH, Fmoc-Thr(tBu)-OH, Fmoc-Cys-(Mmt)-OH, Fmoc-Ala-OH, Fmoc-Val-OH in sequence in sequence , Fmoc-Asn(Trt)-OH, Fmoc-Val-OH, Fmoc-Cys(Ac...
PUM
| Property | Measurement | Unit |
|---|---|---|
| degree of substitution | aaaaa | aaaaa |
| degree of substitution | aaaaa | aaaaa |
Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com