Novel urea type compound preparation method
A compound and new method technology, applied in the field of preparation of urea compounds, can solve the problems of easy oxidation, difficult preparation, high toxicity, etc., and achieve the effect of mild reaction conditions, easy separation and purification, and stable oxidation
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
Embodiment 1
[0023] Embodiment one: synthetic compound ( I - a )
[0024]
[0025] Add 3-bromo-3-benzylindolin-2-one ( II ) (0.1 mmol, 30.2 mg), O -Tosyl- N - Bianoxycarbonyl hydroxylamine ( III ) (0.15 mmol, 48.2 mg), CsCO 3 (0.2 mmol, 38.6 mg), then 2 ml of dichloromethane was added, and the mixture was stirred at room temperature for 14 h. Then p-toluenesulfonic acid (0.25 mmol, 43.1 mg) was added, and the reaction mixture was stirred at room temperature for 8 h. After the reaction was complete, the solvent was evaporated to dryness under reduced pressure to obtain a crude product. The crude product was separated and purified by column chromatography to obtain the compound I - a , White solid, yield 94%.
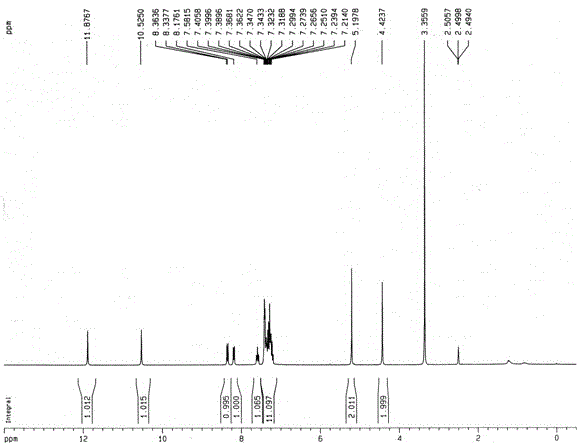
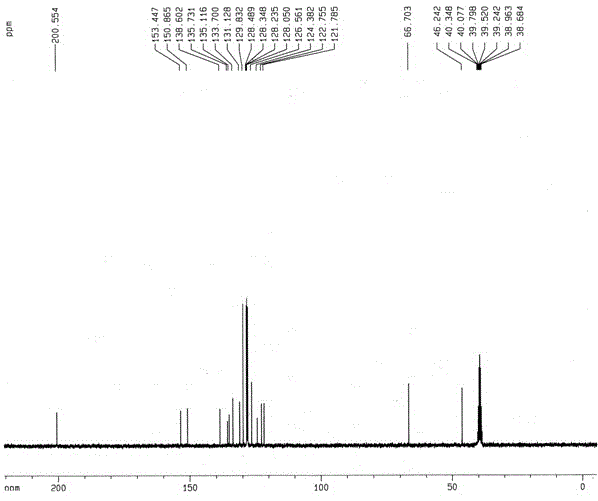
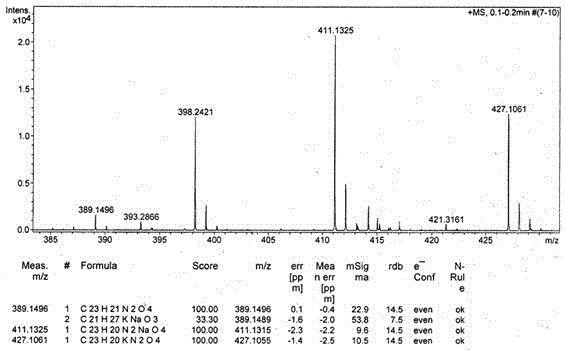
[0026] The resulting compound I-a The hydrogen spectrum, carbon spectrum and mass spectrum data are as follows:
[0027] 1 H NMR (DMSO-d 6 , 300 MHz), δ (ppm): 4.42 (s, 2H), 5.20 (s, 2H), 7.19-7.43 (m, 11H), 7.55-7.61 (m, 1H), 8.17-8.20 (m, 1H), 8.35 (d, J = 7...
Embodiment 2
[0028] Embodiment two: synthetic compound ( I - b )
[0029]
[0030] In a rigid glass tube add 3-bromo-3-(2-methylbenzyl)indolin-2-one ( II ) (0.1 mmol, 31.6 mg), O -Tosyl- N - Bianoxycarbonyl hydroxylamine ( III ) (0.15 mmol, 48.2 mg), K 2 CO 3 (0.2 mmol, 27.6 mg), then 2 ml of acetonitrile was added, and the mixture was stirred at room temperature for 16 h. Then p-toluenesulfonic acid (0.25 mmol, 43.1 mg) was added, and the reaction mixture was continued to stir at room temperature for 8 h. After the reaction was complete, the acetonitrile was evaporated to dryness under reduced pressure to obtain a crude product. The crude product was separated and purified by column chromatography to obtain the compound I - b (white solid, yield 99%).
[0031] The resulting compound I-b The hydrogen spectrum, carbon spectrum and mass spectrum data are as follows:
[0032] 1 H NMR (DMSO-d 6 , 300 MHz), δ (ppm): 2.15 (s, 3H), 4.47 (s, 2H), 5.17 (s, 2H), 7.15-7.18 (m,...
Embodiment 3
[0033] Embodiment three: synthetic compound ( I - c )
[0034]
[0035] Add 3-bromo-3-(3-methylbenzyl)indolin-2-one ( II ) (0.1 mmol, 31.6 mg), O -Tosyl- N - Bianoxycarbonyl hydroxylamine ( III ) (0.15 mmol, 48.2 mg), Et 3 N (0.4 mmol, 40.4 mg), then 2 ml of toluene was added, and the mixture was stirred at room temperature for 30 h. Then p-toluenesulfonic acid (0.25 mmol, 43.1 mg) was added, and the reaction mixture was continued to stir at room temperature for 8 h. After the reaction was complete, the toluene was evaporated to dryness under reduced pressure to obtain a crude product. The crude product was separated and purified by column chromatography to obtain the compound I - c (white solid, yield 92%).
[0036] The resulting compound I - c The hydrogen spectrum, carbon spectrum and mass spectrum data are as follows:
[0037] 1 H NMR (DMSO-d 6 , 300 MHz), δ (ppm): 2.27 (s, 3H), 4.37 (s, 2H), 5.20 (s, 2H), 7.04-7.06 (m, 3H), 7.18-7.23 (m, 2H), 7.34-7...
PUM
 Login to View More
Login to View More Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com