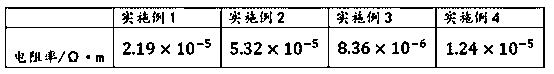Preparation method for electric conduction flake silver-coated copper powder
A technology of flake copper powder and silver-coated copper powder, applied in transportation and packaging, metal processing equipment, coating and other directions, can solve the problems of easy oxidation, high temperature resistance, poor electrical conductivity, etc., and is not easy to be oxidized. , storage stability, good electrical conductivity
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
preparation example Construction
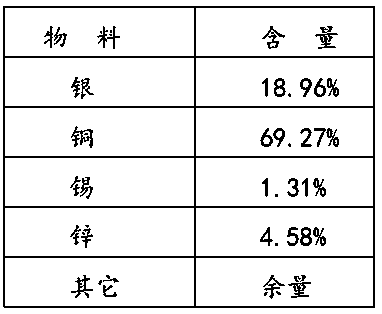
[0016] The preparation method of conductive flake silver-coated copper powder proposed by the present invention adopts tin protochloride to activate copper powder first, forms crystal points on its surface, and makes the surface coating of flake copper powder uniform, to improve the conductivity and stability. The main preparation process is:
[0017] The first step is to clean the surface of the copper powder with an ethanol solution of stannous chloride, and at the same time make the tin ions adsorb to the surface of the copper powder. Using ethanol as the solvent can effectively wash off the oily substances on the surface, and at the same time avoid large areas of copper and water. Contact results in secondary oxidation of the surface.
[0018] The second step uses the tin ions adsorbed on the surface to form a layer of silver crystal points on the copper surface.
[0019] The third step is the oxidation-reduction reaction, which continues to deposit silver on the copper ...
Embodiment 1
[0022] The first step: copper surface cleaning and activation
[0023] Add 20g of flake copper powder into the cleaning solution and stir for 20 minutes. After removing the oxide layer and oily substances on the surface of the flake copper powder, filter, add the filter cake to the reaction kettle, and then add an aqueous organic amine solution to the reaction kettle once, and stir evenly.
[0024] Wherein, the cleaning solution is obtained by adding 1 g of stannous chloride into 130 ml of ethanol and stirring evenly. The primary organic amine aqueous solution is prepared by mixing 75ml of deionized (DI) water and 1g of tetraethylenepentamine.
[0025] The second step: the formation of crystal points on the copper surface
[0026] Add 0.2 g of gum arabic to the secondary organic amine aqueous solution to obtain a mixed solution, and then add the mixed solution into the reaction kettle. Add the silver nitrate solution dropwise into the reaction kettle, continue to react at r...
Embodiment 2
[0033] The first step: copper surface cleaning and activation
[0034] Add 20g of flake copper powder into the cleaning solution and stir for 20 minutes. After removing the oxide layer and oily substances on the surface of the flake copper powder, filter, add the filter cake to the reaction kettle, and then add an aqueous organic amine solution to the reaction kettle once, and stir evenly.
[0035] Wherein, the cleaning solution is obtained by adding 1 g of stannous chloride into 130 ml of ethanol and stirring evenly. The primary organic amine aqueous solution is prepared by mixing 75ml of deionized (DI) water and 1g of tetraethylenepentamine.
[0036] The second step: the formation of crystal points on the copper surface
[0037] Add 0.2 g of gum arabic to the secondary organic amine aqueous solution to obtain a mixed solution, and then add the mixed solution into the reaction kettle. Add the silver nitrate solution dropwise into the reaction kettle, continue to react at r...
PUM
 Login to View More
Login to View More Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com