Method for producing sec-butyl acetate by synthesizing acetic acid and mixed C4
A technology of sec-butyl acetate and acetic acid, which is applied in the chemical industry, can solve problems such as environmental pollution, many side reactions, and long reaction time, and achieve the effects of reducing production costs, good stability, high activity and selectivity
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
Embodiment 1
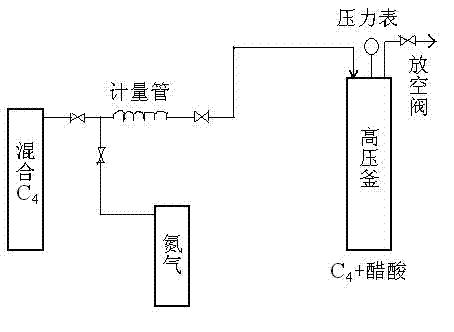
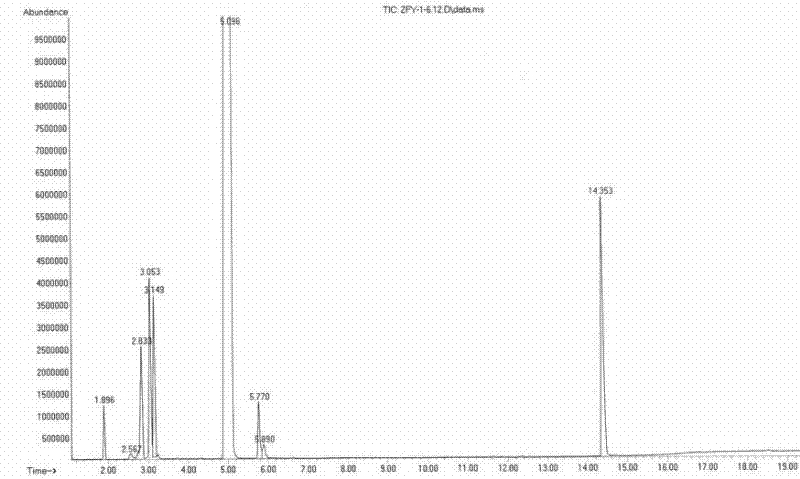
[0032] 2.0mL of resin catalyst and 13mL of glacial acetic acid were charged into the above-mentioned autoclave, and mixed C4 (mass composition was n-butane 11.8%, isobutane 34.6%, 1-butene 17.6%, 2-butene 32.6%, Butadiene, alkyne, etc. 3.4%) 20mL, the molar ratio of n-butene (1-butene, 2-butene) to glacial acetic acid is 0.5:1 (ene-acid ratio), and nitrogen is gradually pressurized to 5.0MPa to adjust the reaction The temperature was raised to 120°C, and the reaction was stopped after 10 hours of reaction, and samples were taken for analysis. The product was analyzed by an Agilent 6890N gas chromatograph. The conversion rate of acetic acid was 90.5%, and the selectivity of sec-butyl acetate was 97.8%.
Embodiment 2
[0034] Except reaction pressure is changed to 6.0MPa, other is the same as embodiment 1, the result is: acetic acid conversion rate 88.5%, sec-butyl acetate selectivity 91.3%.
Embodiment 3
[0036] Except that the reaction temperature was changed to 160° C., the others were the same as in Example 1, and the results were: the conversion rate of acetic acid was 72.3%, and the selectivity of sec-butyl acetate was 77.9%.
[0037] The present invention makes full use of refinery by-product C 4 resources, using strongly acidic styrene-based cation exchange resins as catalysts, studied mixed C 4 (Containing 1-butene, 2-butene, n-butane, isobutane, butadiene, etc.) and glacial acetic acid to synthesize sec-butyl acetate. Through single factor experiment, the effects of reaction time, reaction temperature, reaction pressure, catalyst dosage and molar ratio of enoic acid on the conversion of acetic acid were investigated, and the optimum process conditions for the synthesis of sec-butyl acetate were determined.
PUM
 Login to View More
Login to View More Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com