Method for transforming exogenous mitochondrion into mammal cells
A mammalian and mitochondrial technology, applied in the field of biogenetic engineering, can solve the problems of unclear mechanism, affecting the function of target RNA, unrealized expression and functional identification of artificially synthesized mitochondrial DNA, and achieves the effect of high purity
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
Embodiment 1
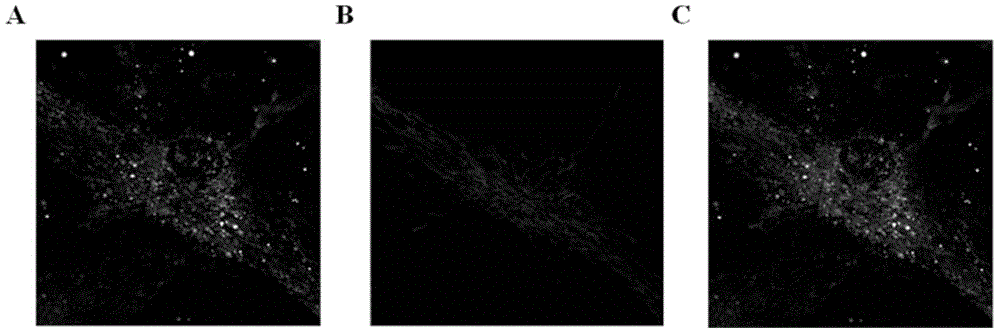
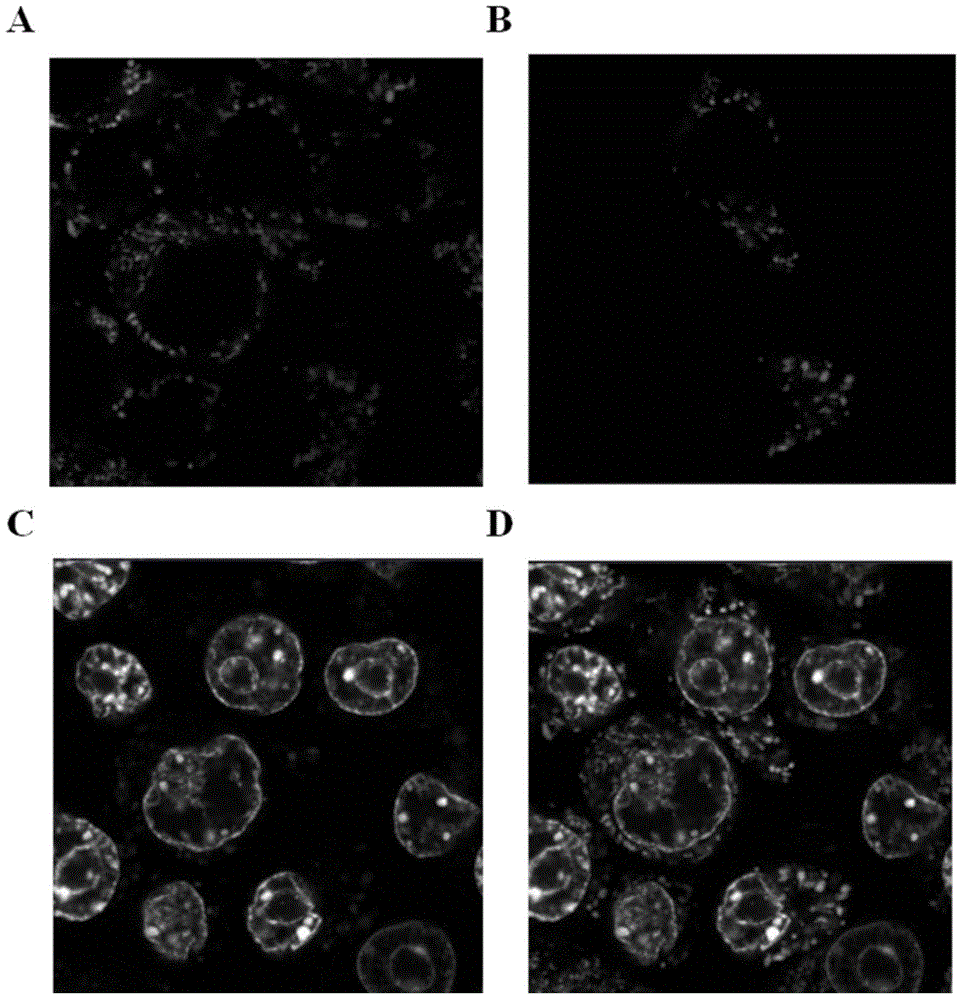
[0049] This example is to prove that exogenous mitochondria can enter mammalian cells through endocytosis. By stably expressing the mitochondrial-localized fluorescent protein EYFP in macrophages, the endogenous mitochondria of macrophages are labeled with EYFP, and the mitochondrial-localized fluorescent protein DsRed2 is stably expressed in NIH3T3 cells, and the mitochondria of NIH3T3 cells are labeled with DsRed2 , Isolate NIH3T3 mitochondria with DsRed2 labeling, add them to the culture system of macrophages, and observe them under a confocal microscope after 12 hours. It can be observed that DsRed2-labeled mitochondria enter into macrophages, which can present the same appearance as that in macrophages. Source consistent morphology of EYFP-tagged mitochondria. Specifically:
[0050] 1. Mouse macrophage cell line RAW264.7 cells and NIH3T3 cell culture medium (high glucose DMEM (purchased from Hyclone, product number: SH30022.01B); 10% fetal bovine serum (purchased from GI...
Embodiment 2
[0061] In this example, the circular mitochondrial DNA of the designed sequence is obtained by gene introduction into mouse mitochondrial DNA, that is, the insertion of GFP and Linker sequences, primer synthesis, and DNA splicing, that is, the circular mitochondrial DNA containing the GFP-COX-I fusion gene .
[0062] 1. Design a new artificial circular mitochondrial DNA:
[0063] This example is to obtain circular mitochondrial DNA by gene introduction into mouse mitochondrial DNA, that is, inserting GFP and Linker sequences, specifically, based on the known mitochondrial DNA sequence of wild-type C57 BL / 6J mice (sequence source : NCBI GenBank: EF108336) and inserted the GFP gene and Linker sequence (specifically as shown in Seq.ID No.1) at the 5328 site, and designed a circular mitochondria containing a GFP-COX-I fusion gene of about 5Kb DNA.
[0064] 2. According to the specific composition of the designed circular mitochondrial DNA, a plurality of 50bp-60bp DNA fragments ...
Embodiment 3
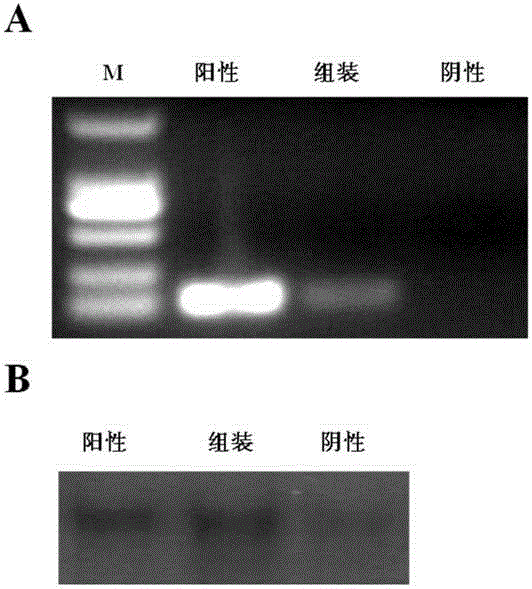
[0070] This example describes the process of assembling the circular mitochondrial DNA obtained in Example 2 with the mitochondrial shell of NIH3T3 Rho0 cells in vitro to make artificially synthesized mitochondria, and the process of extracting and identifying RNA and DNA of artificially synthesized mitochondria . figure 2 Shown is the detection of the correct transcript in the synthetic mitochondria ( figure 2 A) and DNA replication ( figure 2 B).
[0071] 1. Cultivation of Rho0 cells without mitochondrial DNA:
[0072] 1. Add 1.5 μg / mL ditercalinium or 250 ng / mL ethidium bromide, 50 μg / mL uridine (Sigma) and 110 μg / mL sodium pyruvate to the NIH3T3 cell culture medium;
[0073] 2. Continuously cultivate for one month according to the conventional method;
[0074] 3. Culture with NIH3T3 Rho0 medium (high glucose DMEM, 10% fetal bovine serum, 50 μg / mL uridine, 110 μg / mL sodium pyruvate, penicillin and streptomycin double antibodies);
[0075] 4. Collect the cells to obt...
PUM
| Property | Measurement | Unit |
|---|---|---|
| strength | aaaaa | aaaaa |
| capacitance | aaaaa | aaaaa |
| electrical resistance | aaaaa | aaaaa |
Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com